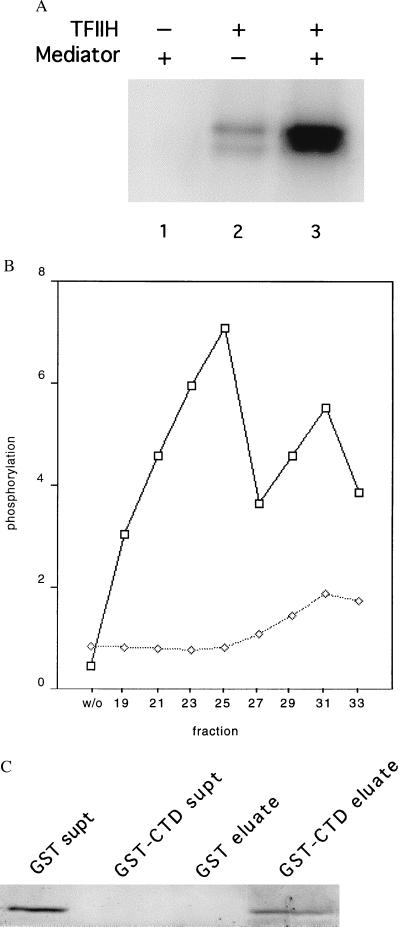
Figure 5.
Physical and functional interaction of mouse Mediator with the RNA polymerase II CTD. (A) Stimulation by mouse Mediator of CTD phosphorylation by TFIIH. Recombinant His-tagged yeast CTD (50 ng) was incubated with rat TFIIH (15 ng) and mouse Mediator (TSK-Heparin-5PW fraction 25, 100 ng) in 15 μl of 20 mM Hepes, pH 7.6/110 mM potassium acetate/5 μM (0.3 μCi) [γ-32P]ATP/7.5 mM magnesium acetate/2 mM DTT for 30 min at 23°C, followed by electrophoresis in an SDS-polyacrylamide gel and autoradiography. (B) Profile of stimulatory activity shown in A across the TSK-Heparin-5PW column (1 μl of each fraction, corresponding to 100 ng of fraction 25, or control without fraction, designated “w/o”), measured in the presence (upper curve) or absence (lower curve) of added TFIIH. Intensities of bands determined with a PhosphorImager are plotted in arbitrary units on the abscissa. (C) Binding of mouse Mediator to GST-CTD. Glutathione-agarose (30 μl of a 50% slurry, Sigma) was incubated with GST (50 μg) or GST-CTD (50 μg) for 1 h at 4°C with gentle agitation. The beads were washed three times with buffer E(600) (20 mM Tris⋅Cl, pH 7.9/1 mM MgCl2/10 μM ZnCl2/0.2 mM EDTA/10% glycerol/protease inhibitors; mM potassium acetate in parenthesis) containing 0.02% Nonidet P-40, and twice in buffer E(200) containing 0.01% Nonidet P-40. The beads then were incubated with Mediator (TSK-Heparin-5PW fraction 25, 1.5 μg) in 100 μl of buffer E(200) containing 0.01% Nonidet P-40 for 2 h at 4°C with gentle agitation, followed by three washes with buffer E(200) containing 0.1% Nonidet P-40 and elution with 35 μl of 10 mM reduced glutathione in 50 mM Tris acetate, pH 7.8, for 10 min at 4°C with gentle agitation. The supernatants from the initial binding of Mediator, concentrated by trichloroacetic acid precipitation (“GST supt,” “GST-CTD supt”), and the entire eluates were analyzed by SDS/PAGE and immunoblotting with anti-mMed7 antibodies.