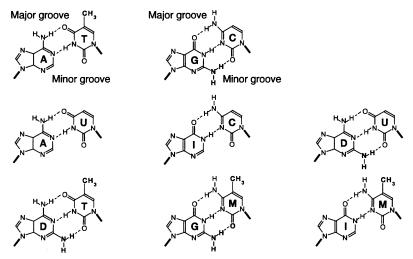
Figure 1.
Structural formulae of the eight different purine⋅pyrimidine base pairs between the natural (A, T, G, C) and exocyclic-modified (U, I, D, M) bases used. A, T, G, and C indicate, respectively, 2′-deoxyadenosine, 2′-deoxythymidine, 2′-deoxyguanosine, and 2′-deoxycytidine, and U, I, D, and M indicate 2′-deoxyuridine, 2′-deoxyinosine, 2,6-diaminopurine-2′-deoxyriboside, and 2′-deoxy-5-methylcytidine. The deoxyribose is represented by a thick bar corresponding to its bonding at positions N9 and N1 of purines and pyrimidines, respectively. The 5-methyl and 2-amino exocyclic groups are shown in bold.