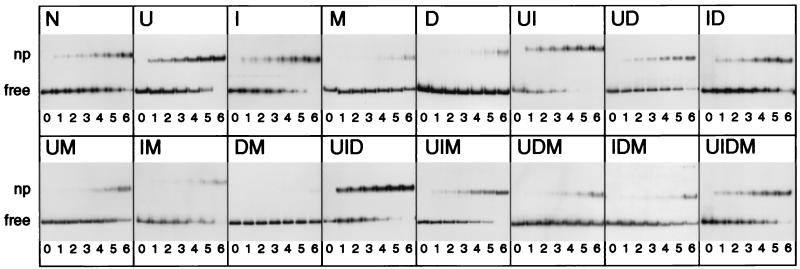
Figure 2.
Addition of DNA exocyclic groups reduces and their removal increases the affinity of the tyrT-containing DNA fragment for histone octamer as determined by a competitor titration experiment using bandshift assays. 5′ end-labeled 160-bp tyrT DNA fragments (17) containing natural or modified bases (Fig. 1) were produced according to a PCR amplification procedure (described in ref. 17 with modifications) designed to minimize the concentration of end-labeled specific DNA in the assembling mixture and to increase its specific activity for binding studies and hydroxyl-radical footprinting, respectively. Nucleosome particles (nps) were assembled on labeled specific DNA by using a salt-dilution protocol (10) with H1-stripped long chromatin acting as a histone octamer donor and different amounts of bulk core-nucleosomal DNA as competitor DNA. Representative gels of the reconstitution of core particles at 22°C from all 16 substituted variants of tyrT are shown. Letters above gels refer to the type and number of substitutions present (see Fig. 1 for reference and abbreviations). Corresponding to numbers 1, 2, 3, 4, 5, and 6 below the gels, 11, 8, 5, 2, 1, and 0 μg of competitor DNA were added before salt dilution to a constant amount of labeled specific DNA and H1-stripped long chromatin. A control experiment also was carried out by salt dilution on DNA alone (lane 0). Positions of 160-bp tyrT DNA fragment either as complexed with histone octamer (np) or unbound DNA (free) are indicated. N indicates unsubstituted DNA.