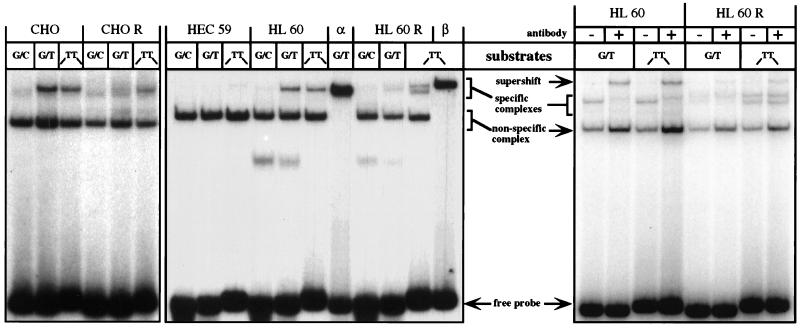
Figure 1.
Mismatch- and IDL-binding activities in extracts of CHO, CHO R, HEC59, HL60, and HL60R cells. The extracts were incubated with radioactively labeled oligonucleotide duplexes (see Materials and Methods) either perfectly complementary (G/C) or containing a single mispair (G/T) or an IDL containing two extrahelical thymines (-TT-) (10). (Left and Center) The bandshift experiments were carried out with cytoplasmic extracts of CHO, CHO R, HEC59, HL60, and HL60R cells. The electrophoretic mobility of the protein/DNA complexes was compared with those formed upon incubation of the oligonucleotide substrates with the purified recombinant hMutSα (lane α) or hMutSβ (lane β). Note that, unlike the purified recombinant hMSH3/hMSH2 heterodimer, the hMutSβ present in human cell extracts gave rise to two distinct protein/DNA complexes (lane HL60R/TT). Neither of these complexes comigrates with hMutSα-bound substrates (e.g., lane HL60R/G/T) (see also text). (Right) Supershift experiments using the anti-hMSH6 mAb 66H6. Addition of the mAb to the binding mixtures containing extracts of HL60 or HL60R cells retarded the mobility of the oligonucleotide probes bound by hMutSα. The figure is an autoradiograph of a nondenaturing 6% polyacrylamide gel run in TAE buffer.