Abstract
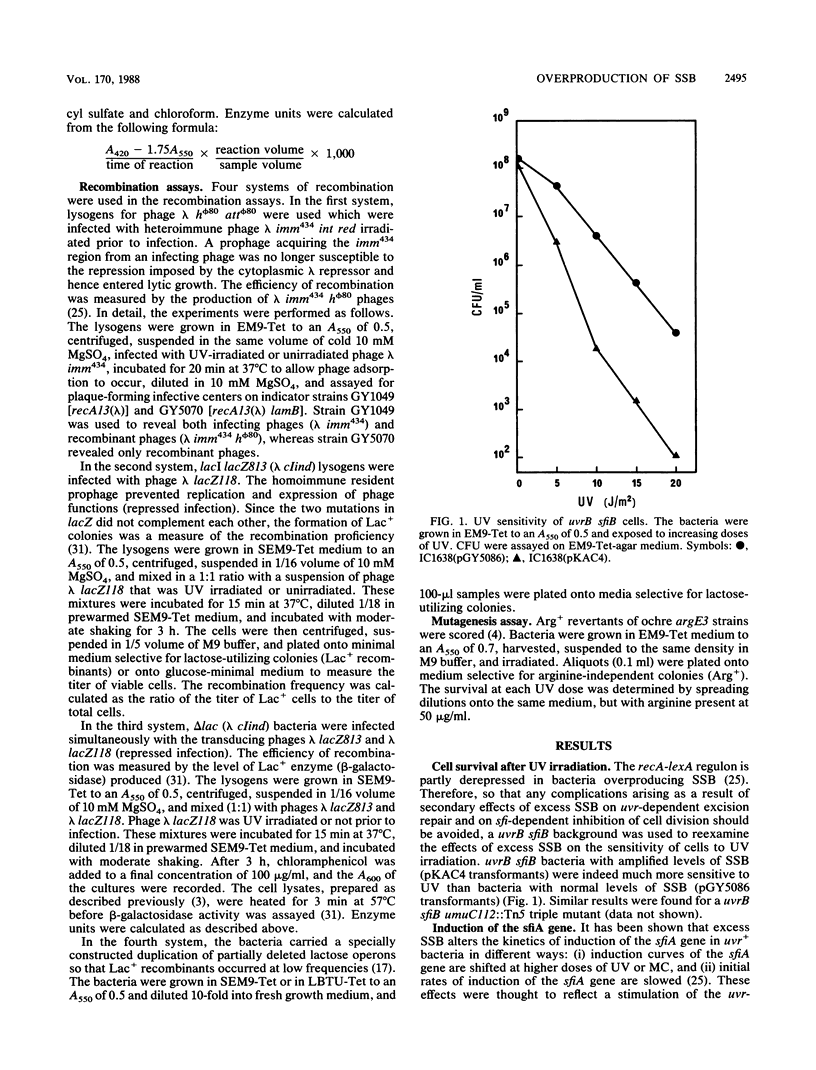
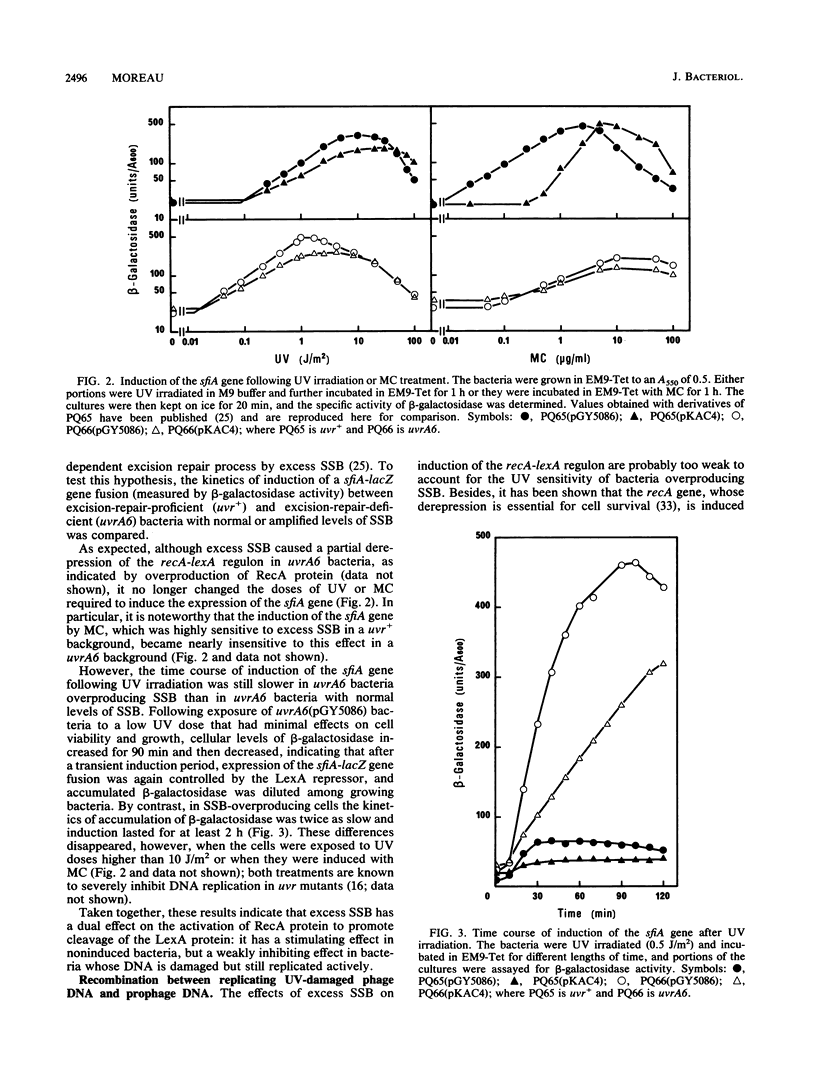
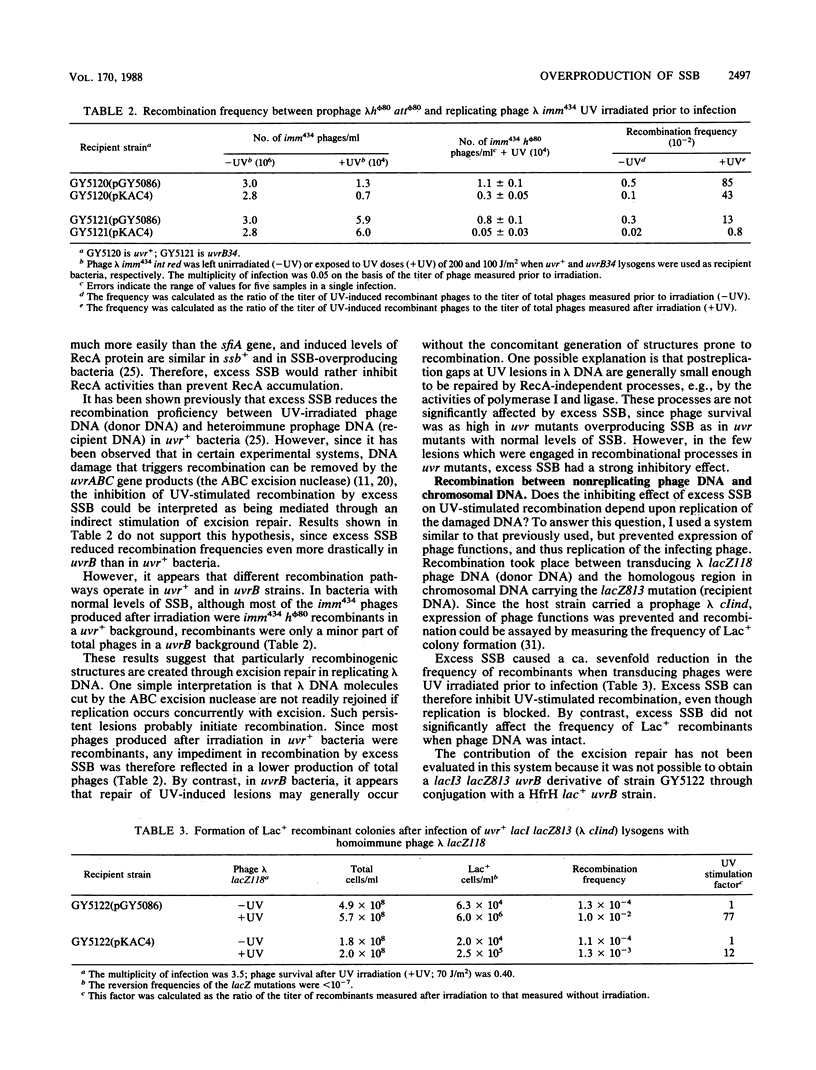
Overproduction of single-stranded DNA (ssDNA)-binding protein (SSB) in uvr Escherichia coli mutants results in a wide range of altered phenotypes. (i) Cell survival after UV irradiation is decreased; (ii) expression of the recA-lexA regulon is slightly reduced after UV irradiation, whereas it is increased without irradiation; and (iii) recombination of UV-damaged lambda DNA is inhibited, whereas recombination of nonirradiated DNA is unaffected. These results are consistent with the idea that in UV-damaged bacteria, SSB is first required to allow the formation of short complexes of RecA protein and ssDNA that mediate cleavage of the LexA protein. However, in a second stage, SSB should be displaced from ssDNA to permit the production of longer RecA-ssDNA nucleoprotein filaments that are required for strand pairing and, hence, recombinational repair. Since bacteria overproducing SSB appear identical in physiological respects to recF mutant bacteria, it is suggested that the RecF protein (alone or with other proteins of the RecF pathway) may help RecA protein to release SSB from ssDNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armengod M. E., Blanco M. Influence of the recF143 mutation of Escherichia coli K12 on prophage lambda induction. Mutat Res. 1978 Oct;52(1):37–47. doi: 10.1016/0027-5107(78)90093-3. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge E. A., Low K. B. Detection of transcribable recombination products following conjugation in rec+, reCB- and recC-strains of Escherichia coli K12. J Mol Biol. 1974 Mar 15;83(4):447–457. doi: 10.1016/0022-2836(74)90506-3. [DOI] [PubMed] [Google Scholar]
- Blanco M., Herrera G., Aleixandre V. Different efficiency of UmuDC and MucAB proteins in UV light induced mutagenesis in Escherichia coli. Mol Gen Genet. 1986 Nov;205(2):234–239. doi: 10.1007/BF00430433. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4193–4197. doi: 10.1073/pnas.82.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Murphy J. B., Whittier R. F., Lorensen E., Sninsky J. J. Amplification of ssb-1 mutant single-stranded DNA-binding protein in Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):193–211. doi: 10.1016/0022-2836(83)90075-x. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Williams K. R. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- Cohen S. P., Resnick J., Sussman R. Interaction of single-strand binding protein and RecA protein at the single-stranded DNA site. J Mol Biol. 1983 Jul 15;167(4):901–909. doi: 10.1016/s0022-2836(83)80119-3. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Hanawalt P. C. Effect of a lexA41(Ts) mutation on DNA repair in recA(Def) derivatives of Escherichia coli K-12. Mol Gen Genet. 1985;201(3):387–392. doi: 10.1007/BF00331328. [DOI] [PubMed] [Google Scholar]
- Golub E. I., Low K. B. Indirect stimulation of genetic recombination. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1401–1405. doi: 10.1073/pnas.80.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., West S. C., Stasiak A. Role of RecA protein spiral filaments in genetic recombination. Nature. 1984 May 17;309(5965):215–219. doi: 10.1038/309215a0. [DOI] [PubMed] [Google Scholar]
- Julin D. A., Riddles P. W., Lehman I. R. On the mechanism of pairing of single- and double-stranded DNA molecules by the recA and single-stranded DNA-binding proteins of Escherichia coli. J Biol Chem. 1986 Jan 25;261(3):1025–1030. [PubMed] [Google Scholar]
- Karu A. E., Belk E. D. Induction of E. coli recA protein via recBC and alternate pathways: quantitation by enzyme-linked immunosorbent assay (ELISA). Mol Gen Genet. 1982;185(2):275–282. doi: 10.1007/BF00330798. [DOI] [PubMed] [Google Scholar]
- Kato T., Rothman R. H., Clark A. J. Analysis of the role of recombination and repair in mutagenesis of Escherichia coli by UV irradiation. Genetics. 1977 Sep;87(1):1–18. doi: 10.1093/genetics/87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khidhir M. A., Casaregola S., Holland I. B. Mechanism of transient inhibition of DNA synthesis in ultraviolet-irradiated E. coli: inhibition is independent of recA whilst recovery requires RecA protein itself and an additional, inducible SOS function. Mol Gen Genet. 1985;199(1):133–140. doi: 10.1007/BF00327522. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. Novel mutants of Escherichia coli that accumulate very small DNA replicative intermediates. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2150–2154. doi: 10.1073/pnas.72.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad E. B. Method for the isolation of Escherichia coli mutants with enhanced recombination between chromosomal duplications. J Bacteriol. 1977 Apr;130(1):167–172. doi: 10.1128/jb.130.1.167-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczykowski S. C., Krupp R. A. Effects of Escherichia coli SSB protein on the single-stranded DNA-dependent ATPase activity of Escherichia coli RecA protein. Evidence that SSB protein facilitates the binding of RecA protein to regions of secondary structure within single-stranded DNA. J Mol Biol. 1987 Jan 5;193(1):97–113. doi: 10.1016/0022-2836(87)90630-9. [DOI] [PubMed] [Google Scholar]
- Lin P. F., Howard-Flanders P. Genetic exchanges caused by ultraviolet photoproducts in phage lambda DNA molecules: the role of DNA replication. Mol Gen Genet. 1976 Jul 23;146(2):107–115. doi: 10.1007/BF00268079. [DOI] [PubMed] [Google Scholar]
- Lu C., Scheuermann R. H., Echols H. Capacity of RecA protein to bind preferentially to UV lesions and inhibit the editing subunit (epsilon) of DNA polymerase III: a possible mechanism for SOS-induced targeted mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):619–623. doi: 10.1073/pnas.83.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Walker G. C. New phenotypes associated with mucAB: alteration of a MucA sequence homologous to the LexA cleavage site. J Bacteriol. 1987 May;169(5):1818–1823. doi: 10.1128/jb.169.5.1818-1823.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P. L. Effects of overproduction of single-stranded DNA-binding protein on RecA protein-dependent processes in Escherichia coli. J Mol Biol. 1987 Apr 20;194(4):621–634. doi: 10.1016/0022-2836(87)90239-7. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Roberts J. W. RecA protein--promoted lambda repressor cleavage: complementation between RecA441 and RecA430 proteins in vitro. Mol Gen Genet. 1984;198(2):25–34. doi: 10.1007/BF00328696. [DOI] [PubMed] [Google Scholar]
- Moreau P., Bailone A., Devoret R. Prophage lambda induction of Escherichia coli K12 envA uvrB: a highly sensitive test for potential carcinogens. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3700–3704. doi: 10.1073/pnas.73.10.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousset S., Thomas R. Dilysogenic excision: an accessory expression of the termination function? Cold Spring Harb Symp Quant Biol. 1968;33:749–754. doi: 10.1101/sqb.1968.033.01.085. [DOI] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Helicase properties of the Escherichia coli UvrAB protein complex. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3638–3642. doi: 10.1073/pnas.84.11.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K. R., Mount D. W. Differential repression of SOS genes by unstable lexA41 (tsl-1) protein causes a "split-phenotype" in Escherichia coli K-12. J Mol Biol. 1987 Jan 5;193(1):27–40. doi: 10.1016/0022-2836(87)90623-1. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Lark M. W., Low K. B. Specialized transduction with lambda plac5: dependence on recA and on configuration of lac and att lambda. J Virol. 1981 May;38(2):497–503. doi: 10.1128/jvi.38.2.497-503.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., McLaughlin T., Low B. Transduction versus "conjuduction": evidence for multiple roles for exonuclease V in genetic recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1043–1047. doi: 10.1101/sqb.1979.043.01.113. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Hofnung M. The SOS Chromotest, a colorimetric bacterial assay for genotoxins: procedures. Mutat Res. 1985 Jun;147(3):65–78. doi: 10.1016/0165-1161(85)90020-2. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Moreau P. L., Ginsburg H., Mount D. W., Devoret R. Cell survival, UV-reactivation and induction of prophage lambda in Escherichia coli K12 overproducing RecA protein. Mol Gen Genet. 1982;188(1):37–43. doi: 10.1007/BF00332993. [DOI] [PubMed] [Google Scholar]
- Radman M., Cordone L., Krsmanovic-Simic D., Errera M. Complementary action of recombination and excision in the repair of ultraviolet irradiation damage to DNA. J Mol Biol. 1970 Apr 14;49(1):203–212. doi: 10.1016/0022-2836(70)90386-4. [DOI] [PubMed] [Google Scholar]
- Resnick J., Sussman R. Escherichia coli single-strand DNA binding protein from wild type and lexC113 mutant affects in vitro proteolytic cleavage of phage lambda repressor. Proc Natl Acad Sci U S A. 1982 May;79(9):2832–2835. doi: 10.1073/pnas.79.9.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Phizicky E. M., Burbee D. G., Roberts C. W., Moreau P. L. A brief consideration of the SOS inducing signal. Biochimie. 1982 Aug-Sep;64(8-9):805–807. doi: 10.1016/s0300-9084(82)80133-8. [DOI] [PubMed] [Google Scholar]
- SIGNER E. R. RECOMBINATION BETWEEN COLIPHAGES LAMBDA AND PHI-80. Virology. 1964 Apr;22:650–651. doi: 10.1016/0042-6822(64)90090-x. [DOI] [PubMed] [Google Scholar]
- Smith C. L. Response of recA-dependent operons to different DNA damage signals. J Biol Chem. 1985 Aug 25;260(18):10069–10074. [PubMed] [Google Scholar]
- Smith T. A., Hays J. B. Repair and recombination of nonreplicating UV-irradiated phage DNA in E. coli II. Stimulation of RecF-dependent recombination by excision repair of cyclobutane pyrimidine dimers and of other photoproducts. Mol Gen Genet. 1985;201(3):393–401. doi: 10.1007/BF00331329. [DOI] [PubMed] [Google Scholar]
- Tessman E. S., Tessman I., Peterson P. K., Forestal J. D. Roles of RecA protease and recombinase activities of Escherichia coli in spontaneous and UV-induced mutagenesis and in Weigle repair. J Bacteriol. 1986 Dec;168(3):1159–1164. doi: 10.1128/jb.168.3.1159-1164.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms B., Wackernagel W. Regulatory role of recF in the SOS response of Escherichia coli: impaired induction of SOS genes by UV irradiation and nalidixic acid in a recF mutant. J Bacteriol. 1987 Apr;169(4):1731–1736. doi: 10.1128/jb.169.4.1731-1736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Hartke M. A. Suppression of Escherichia coli recF mutations by recA-linked srfA mutations. J Bacteriol. 1984 Feb;157(2):498–506. doi: 10.1128/jb.157.2.498-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert M. R., Margossian L. J., Clark A. J. Two-component suppression of recF143 by recA441 in Escherichia coli K-12. J Bacteriol. 1984 Nov;160(2):702–705. doi: 10.1128/jb.160.2.702-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Wang W. B., Tessman E. S. Location of functional regions of the Escherichia coli RecA protein by DNA sequence analysis of RecA protease-constitutive mutants. J Bacteriol. 1986 Nov;168(2):901–910. doi: 10.1128/jb.168.2.901-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey S. D., Porter R. D. General recombination in Escherichia coli K-12: in vivo role of RecBC enzyme. J Bacteriol. 1985 Apr;162(1):29–34. doi: 10.1128/jb.162.1.29-34.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieg J., Kushner S. R. Analysis of genetic recombination between two partially deleted lactose operons of Escherichia coli K-12. J Bacteriol. 1977 Jul;131(1):123–132. doi: 10.1128/jb.131.1.123-132.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


