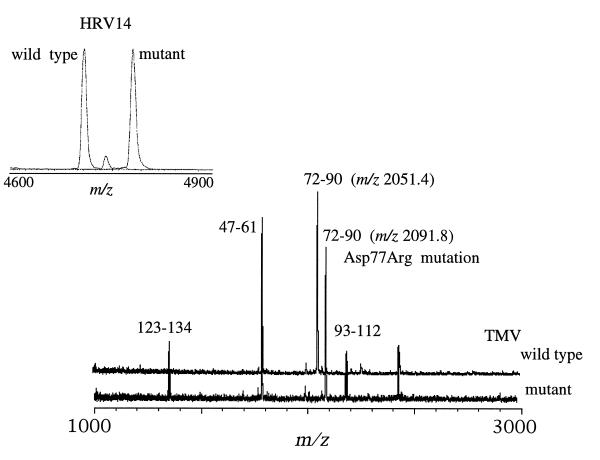
Figure 2.
Comparison of the MALDI-TOF mass spectra resulting from the trypsin digestion of wild-type TMV and its mutant TMV-Asp50Arg. Most ion signals are common to both spectra, with the obvious exception of the signals observed at m/z 2050.5 Da in the wild-type spectrum and m/z 2091.8 Da in the mutant’s spectrum. Both signals represent residues 72–90 with the incorporation of the Asp → Arg amino acid change in the mutant. The mutation produces an ion signal (m/z 2091.8) 41 Da higher in mass than the corresponding signal from the wild-type virus. (Inset) Spectra from the trypsin digests of wild-type HRV14 and its naturally occurring mutant HRV14-Cys1199Tyr. Similarly with the HRV14 spectra, most fragments are common to both spectra, with the exception of the ion signal at m/z 4700.5 Da and 4783.8 Da in the HRV14 and HRV14-Cys1199Tyr spectra, respectively. Both ion signals corresponds to residues 187–227 of the VP1 capsid protein, and the mass difference of 83 Da between the two variants is consistent with a Cys → Tyr mutation. Mass analyses of the digestion mixtures resulted in the localization of the mutations in only a matter of minutes (<20 min, including the digestion).