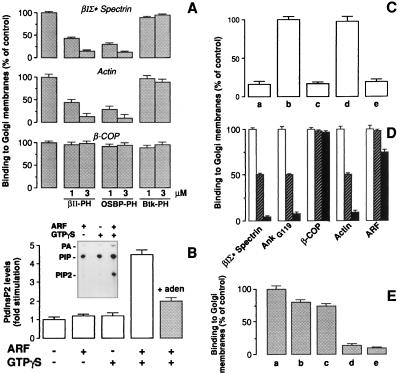
Figure 4.
ARF stimulation increases PtdInsP2 in Golgi fractions, and PtdInsP2 is required for the binding of βIΣ* spectrin. (A) Golgi fractions were incubated with cytosol in the presence of 20 μM GTPγS and 3 μM GST or the indicated concentrations of GST-fused polypeptides containing the PH domains of βII spectrin (βII-PH), OSBP (OSBP-PH), or Btk (Btk-PH). Results (binding to Golgi) are expressed as percent of control binding (GST alone). Data represent the average of four experiments (±1 SD). (B) Golgi were incubated in two steps: first (as indicated) with control buffer or 1 μM ARF or with 20 μM GTPγS alone or GTPγS and 1 μM ARF for 15 min at 37°C. In the second step, membranes were pelleted, rinsed, and incubated with cytosol and 2 μCi/sample of ATP-γ [32P]. Where indicated (filled bar), 2.5 mM adenosine was added during both the first and second incubation steps to block PtdIns4 kinase activity. At the end of the incubation, phospholipids were extracted and analyzed by TLC. The level of 32P-labeled PtdInsP2 is expressed relative to unstimulated control. Data represent the average of eight experiments (error bars ±1 SD). (Inset) A representative TLC with the positions of phosphatidic acid (PA), PtdIns4P (PIP), and PtdIns4,5P2 (PIP2) standards marked. (C) Golgi fractions were incubated with control buffer (a and e), 20 μM GTPγS (b), or 20 μM GTPγS and 250 μM neomycin (c and d). Membranes were pelleted, incubated for 30 min at 4°C with phospholipid liposomes containing phosphatidyl-ethanolamine (PE, a–c) or PtdInsP2/PE (1:5 mol/mol) (d and e), and incubated with cytosol for 15 min at 37°C. Results (βIΣ* spectrin binding to Golgi) are expressed as percent of controls (GTPγS alone). The data represent the average of four experiments (±1 SD). (D) Golgi fractions were incubated in the first step with 1 μM ARF and 20 μM GTPγS, then pelleted, rinsed, and incubated with cytosol in the second step. The buffer used in both steps contained 500 μM ATP (empty bars), 500 μM ATP and 2.5 mM adenosine (striped bars), or no ATP (filled bars). Results (binding to Golgi) are expressed as percent of controls (500 μM ATP alone), averaged over four experiments (error bars ±1 SD). (E) Golgi were incubated with GTPγS and ARF under control conditions (a), in the presence of 1% 1-butanol (b), or 2-butanol (inactive as PLD substrate) (c). Membranes were pelleted and incubated with cytosol and 1-butanol (b) or 2-butanol (c). Golgi fractions were incubated with 0.2 units/ml of PLD from Streptomyces chromofuscus in the absence (d) or in the presence of 100 μM Ca2+ (e), pelleted, rinsed, and incubated with cytosol and PLD in the second step in the absence (d) or in the presence of 1 μM Ca2+ (e). In parallel experiments (not shown) the levels of phosphatidic acid levels were measured as in B, and a 10-fold increase in phosphatidic acid was measured in samples treated with exogenous PLD compared with control samples. Results (βIΣ* spectrin binding to Golgi) are expressed as percent of controls (ARF and GTPγS). Each data represent the average of four experiments (±1 SD).