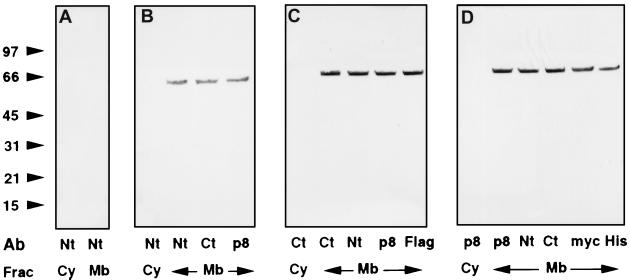
Figure 1.
Localization of ARD1 by Western blotting. Nontransfected NIH 3T3 cells (A) or cells transfected with pcDNA3.1(ARD1) (B), pcDNA3.1(ARD1–FLAG) (C), or pcDNA3.1/myc–His(ARD1) (D) were scraped from three 100-mm dishes in 2 ml of ice-cold buffer A [20 mM Tris (pH 7.4), 1 mM EDTA, 0.32 M sucrose, 5 μg/ml each of aprotinin, and leupeptin, 2 μg/ml pepstatin, and 1 mM 4-(2-aminoethyl)-benzenesulfonyl fluoride] and lysed by 15 passages through a 25-gauge needle. Crude membrane and cytosolic fractions were recovered after centrifugation (100,000 × g, 30 min) of the postnuclear supernatant. Samples of cytosol (Cy) or total membrane (Mb) proteins (40 μg) were separated by SDS/PAGE in 4–20% gels and transferred to nitrocellulose membranes for Western blotting. Primary antibodies indicated below each lane were diluted as follows: anti-Nt-ARD1, 1:100,000; anti-Ct-ARD1, 1:100,000; anti-recombinant ARD1 (p8) 1:15,000; anti-FLAG, 1:2,000; anti-myc, 1:10,000, and anti-His6 C-terminal (His), 1:2,000. Alkaline phosphatase-conjugated anti-mouse and anti-rabbit secondary antibodies were diluted 1:3000. The experiment was repeated once with COS 7 cells and identical results were obtained.