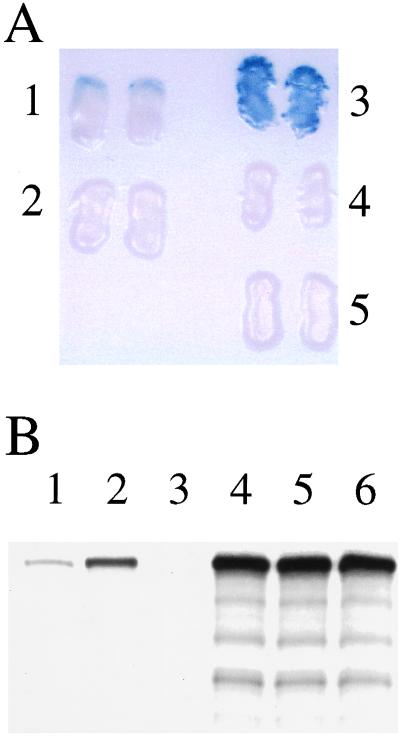
Figure 4.
The high-frequency G843D mutation in PEX1 attenuates the interaction between PEX1 and PEX6. Two-hybrid reporter strains coexpressing G4BD–PEX6 and G4AD–PEX1/G843D (A, lane 1) displayed significantly less β-galactosidase activity by filter assay than strains coexpressing G4BD–PEX6 and G4AD–PEX1 (A, lane 3). Control strains expressing G4BD and G4AD–PEX1/G843D (A, lane 2), G4BD and G4AD–PEX1 (A, lane 4), or G4BD–PEX6 and G4AD (A, lane 5) did not display detectable β-galactosidase activity. Similarly, SDS/PAGE and fluorography (B) revealed that anti-myc immunoprecipitates from lysates containing [35S]-labeled PEX6 and PEX1/G843D-3xmyc (B, lane 1) contained 70% less PEX6 than immunoprecipitates from lysates containing [35S]-labeled PEX6 and PEX1–3xmyc (B, lane 2). Once again, PEX6 was not detected in the anti-myc immunoprecipitate of lysates containing [35S]-labeled PEX6 and untagged PEX1 (B, lane 3). Equal amounts of PEX6 (B, lanes 4–6, respectively) were present in these three lysates before immunoprecipitation with anti-myc antibodies.