Figure 3.
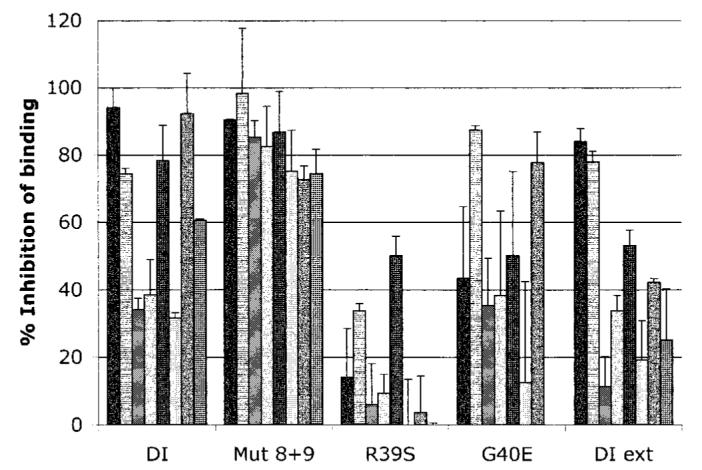
Binding of polyclonal IgG from patients with antiphospholipid syndrome to mutant recombinant human domain I (DI) in the fluid phase. The graph shows the degree to which wild-type and mutant recombinant human domain I (at 3.25 μM) can inhibit polyclonal IgG antiphospholipid antibodies (aPL) from binding to whole β2-glycoprotein I coated on cardiolipin. Variable inhibition was observed with wild-type recombinant human domain I and extended domain I (DI ext). The domain I mutant in which both D residues were mutated (Mut 8+9) had the effect of enhancing binding to the majority of aPL tested. In contrast, the R39S mutation resulted in a significant reduction in binding as compared with wild-type (P = 0.001). G40E had a variable effect. Values are the mean and SD of 8 samples.
