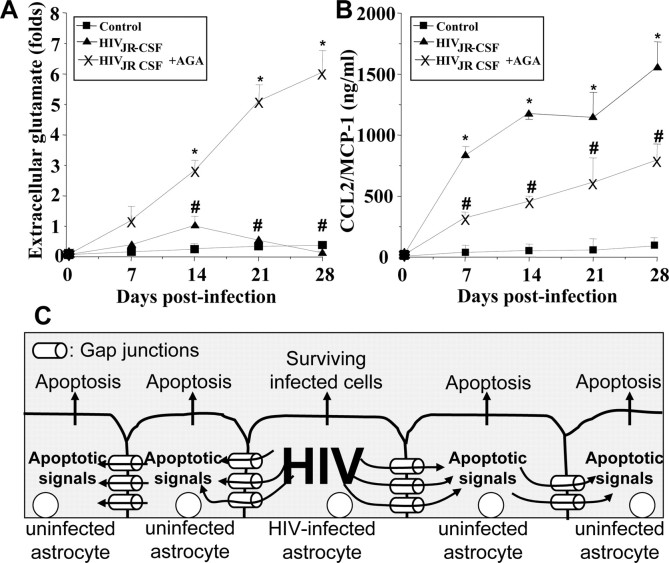
Figure 4.
HIV infection of astrocytes alters glutamate metabolism and CCL2 secretion by a gap junction-dependent mechanism. To examine the functional consequences of HIV infection in astrocytes and the role of gap junctions in control extracellular levels of glutamate and CCL2, quantification of extracellular levels of glutamate and CCL2 was performed by ELISA. A, Extracellular levels of glutamate from uninfected astrocytes were stable during the time course examined (■), and HIV infection of cultures of astrocytes with JR-CSF (▴) virus (HIVADA and HIV92UG021 results are presented in the supplemental material, available at www.jneurosci.org) resulted in a time-dependent accumulation of extracellular glutamate in the media compared with control conditions (*p < 0.0002; n = 9). The gap junction blocker, AGA (×), reduced the increase in extracellular glutamate triggered by HIV infection (#p < 0.0005 compared with viral infection alone; n = 9). B, CCL2 secretion was highly increased by HIV infection, and addition of AGA to HIV-infected cultures resulted in ∼50% reduction in the amount of CCL2 secretion induced by HIV infection alone (*p < 0.0002 compared with control conditions, and # p < 0.0002 compared with HIV infection alone; n = 9). These results indicate that HIV infection of just a few astrocytes results in dysregulation of glutamate metabolism and enhanced secretion of CCL2 into the extracellular medium by a gap junction-dependent mechanism. C, Proposed model illustrating that HIV-infected astrocytes trigger apoptosis in uninfected astrocytes. Proapoptotic signals are generated in HIV-infected astrocytes that diffuse through gap junction channels to uninfected cells, resulting in apoptosis of uninfected astrocytes in close contact with HIV-infected cells.