Abstract
When isogenic strains of Escherichia coli, RR1 (rec+) and HB101 (recA), were transformed with mapped recombinant plasmids known to contain Vibrio harveyi luciferase genes and large regions of DNA flanking on both sides, a small percentage (0.005%) of the colonies expressed high levels of luminescence (up to 10(12) quanta s-1 ml-1) in the absence of added aldehyde. The altered ability to express light was found to be due to a mutation in the host and not to an alteration in the recombinant DNA. When these bright colonies were cured of plasmid, they could be retransformed with cloned V. harveyi gene fragments in cis and in trans to yield luminescent colonies at 100% frequency. The maximum length of V. harveyi DNA required to produce light-emitting E. coli was shorter (6.3 kilobase pairs) than that required for expression of the V. fischeri system in E. coli. Cell extracts from bright clones contained wild-type levels of activity for the heteropolymeric (alpha beta) luciferase; fatty acid labeling revealed the presence of the three acylated polypeptides of the fatty acid reductase system which is involved in aldehyde biosynthesis for the luminescence reaction. The increased light emission in the mutant bacteria appeared to arise in part from production of higher levels of polycistronic mRNAs coding for luciferase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. O., Berends T., Bunch T. A., Holzman T. F., Rausch S. K., Shamansky L., Treat M. L., Ziegler M. M. Cloning of the luciferase structural genes from Vibrio harveyi and expression of bioluminescence in Escherichia coli. Biochemistry. 1984 Jul 31;23(16):3663–3667. doi: 10.1021/bi00311a014. [DOI] [PubMed] [Google Scholar]
- Belas R., Mileham A., Cohn D., Hilman M., Simon M., Silverman M. Bacterial bioluminescence: isolation and expression of the luciferase genes from Vibrio harveyi. Science. 1982 Nov 19;218(4574):791–793. doi: 10.1126/science.10636771. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Boylan M., Graham A. F., Meighen E. A. Functional identification of the fatty acid reductase components encoded in the luminescence operon of Vibrio fischeri. J Bacteriol. 1985 Sep;163(3):1186–1190. doi: 10.1128/jb.163.3.1186-1190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D., Meighen E. Vibrio harveyi aldehyde dehydrogenase. Partial reversal of aldehyde oxidation and its possible role in the reduction of fatty acids for the bioluminescence reaction. J Biol Chem. 1984 Jun 10;259(11):7109–7114. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Mileham A. J., Simon M. I., Nealson K. H., Rausch S. K., Bonam D., Baldwin T. O. Nucleotide sequence of the luxA gene of Vibrio harveyi and the complete amino acid sequence of the alpha subunit of bacterial luciferase. J Biol Chem. 1985 May 25;260(10):6139–6146. [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Simon M., Silverman M. Measuring gene expression with light. Science. 1985 Mar 15;227(4692):1345–1347. doi: 10.1126/science.2983423. [DOI] [PubMed] [Google Scholar]
- Evans J. F., McCracken S., Miyamoto C. M., Meighen E. A., Graham A. F. In vitro synthesis of subunits of bacterial luciferase in an Escherichia coli system. J Bacteriol. 1983 Jan;153(1):543–545. doi: 10.1128/jb.153.1.543-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller F. A family of cloning vectors containing the lacUV5 promoter. Gene. 1982 Jul-Aug;19(1):43–54. doi: 10.1016/0378-1119(82)90187-1. [DOI] [PubMed] [Google Scholar]
- Gunsalus-Miguel A., Meighen E. A., Nicoli M. Z., Nealson K. H., Hastings J. W. Purification and properties of bacterial luciferases. J Biol Chem. 1972 Jan 25;247(2):398–404. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Michaliszyn G. A., Meighen E. A. Induced polypeptide synthesis during the development of bacterial bioluminescence. J Biol Chem. 1976 May 10;251(9):2541–2549. [PubMed] [Google Scholar]
- Miyamoto C. M., Graham A. D., Boylan M., Evans J. F., Hasel K. W., Meighen E. A., Graham A. F. Polycistronic mRNAs code for polypeptides of the Vibrio harveyi luminescence system. J Bacteriol. 1985 Mar;161(3):995–1001. doi: 10.1128/jb.161.3.995-1001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
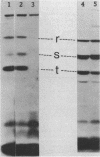
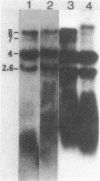
- Wall L. A., Byers D. M., Meighen E. A. In vivo and in vitro acylation of polypeptides in Vibrio harveyi: identification of proteins involved in aldehyde production for bioluminescence. J Bacteriol. 1984 Aug;159(2):720–724. doi: 10.1128/jb.159.2.720-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]