Abstract
Background
Thyrotropin (TSH)‐secreting pituitary adenomas (TSHomas) are rare tumours that can be invasive. It has been suggested that thyroid surgery or radioiodine treatment should not be considered in patients with such tumours as these treatments may facilitate rapid and aggressive tumour expansion.
Aim
To study the effects of thyroid ablative treatment on tumour size and thyroid status in two patients with TSHomas in whom the size of the adenoma was clearly documented before treatment was started.
Methods
Patients studied were: (1) a female patient with a TSHoma who declined to undergo pituitary surgery and underwent a total thyroidectomy instead and (2) a male patient who opted for radioiodine treatment for his recurrent TSHoma. Changes in tumour size on serial magnetic resonance imaging scans, and restoration of euthyroidism were studied.
Results
No marked changes in tumour size or features of aggressiveness occurred in these patients over periods of 8 and 12 years. Euthyroidism was restored and maintained in both patients.
Conclusions
Ablative thyroid treatment can be a safe and successful option to treat TSHomas, but long‐term and close follow‐up of these patients is mandatory to ensure that the size and behaviour of the tumours do not change markedly.
Thyrotropin (TSH)‐producing pituitary tumours are rare tumours that can be invasive. It has been suggested that thyroid surgery, thyroid drugs or radioiodine treatment should not be considered in patients with these tumours as these treatments may facilitate rapid and aggressive tumour expansion.1
We report on the first two patients with TSH‐secreting pituitary tumours, who were successfully and safely treated with thyroid ablative treatment and followed up for 8 and 12 years.
Case 1
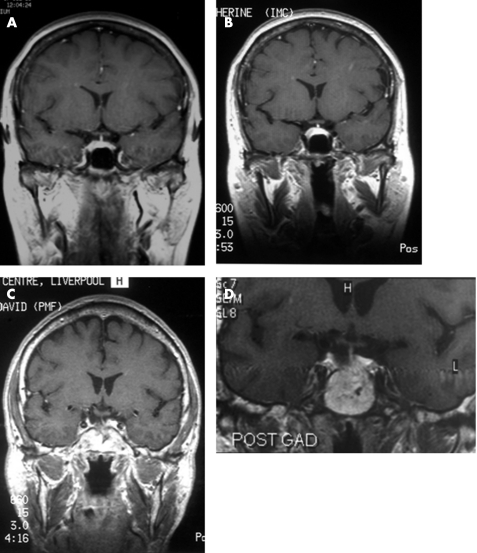
A 32‐year‐old female patient presented in March 1994 with palpitations and complaining of weight loss. She was clinically thyrotoxic; her serum level of free thyroxine (FT4) was raised (66 pmol/l; normal 9.5–23.5 pmol/l) and her serum level of thyroid‐secreting hormone (TSH) was within the normal range (3.92 mIU/l; normal 0.3–5.5 mIU/l). She was treated with carbimazole 40 mg and thyroxine 50 μg daily and became clinically euthyroid. After 16 months, serum levels were as follows: TSH, 17 mIU/l; FT4, 18.4 pmol/l; free tri‐iodothyronine (FT3), 4.7 pmol/l (normal 3.5–6.5 pmol/l). Thyroid autoantibodies were negative. Carbimazole and thyroxine were discontinued and the levels 3 weeks later were as follows: FT4, 44.0 pmol/l; FT3, 12.0 pmol/l; TSH, 4.1 mIU/l; follicle‐stimulating hormone (FSH), 6 IU/l; leutinising hormone, 1.9 IU/l; prolactin, 101 mIU/l (normal <500 mIU/l); and α‐subunit (αSU) 1.5 IU/l (normal <0.5 IU/l), giving a calculated αSU:TSH molar ratio of 10.2. These results were compatible with a diagnosis of hyperthyroidism with inappropriate TSH secretion. After taking 200 μg thyrotropin‐releasing hormone intravenously, TSH rose slightly from 3.98 to 6.04 mIU/l at 20 min, but αSU remained unchanged at 1.4 IU/l. Thyroid hormone tests for her parents, siblings and two children were all normal. Analysis of the thyroid hormone receptor β gene failed to disclose any sequence abnormalities, making the diagnosis of partial resistance to thyroid hormone unlikely. A magnetic resonance imaging (MRI) scan of her pituitary showed an apparent area of low signal intensity on the right side, in keeping with the presence of a pituitary microadenoma (fig 1A).
Figure 1 (A,B) Case 1. Thyrotropin (TSH)‐secreting pituitary microadenoma. (A) Coronal T1‐weighted magnetic resonance image after the administration of gadolinium, obtained in 1995, showing an area of low signal intensity on the right side of the pituitary gland. The patient underwent a total thyroidectomy in 1997. (B) The coronal T1‐weighted post‐gadolinium image on the right was obtained in 2003 and shows a 7×8‐mm intrasellar enhancing mass on the right side of the pituitary gland. (C,D) Case 2. TSH‐secreting pituitary adenoma. Trans‐sphenoidal surgery in 1991 and radioiodine treatment in 1993. (C) Coronal T1‐weighted MRI scan obtained in 1997, showing some residual adenomatous material in the pituitary fossa on the right side that enhances after gadolinium administration. (D) The coronal T1‐weighted image on the right was obtained in 2003 and shows a 6×5‐mm mass in the right side of the pituitary fossa that enhanced with gadolinium administration (POST GAD).
The above data were compatible with a diagnosis of a pituitary TSH‐producing tumour. The patient declined trans‐sphenoidal surgery and opted for a total thyroidectomy, which was carried out in August 1997. After thyroidectomy, she required a daily dose of 150 μg thyroxine to remain clinically euthyroid and to maintain her FT4 and FT3 levels within normal limits. Her TSH levels continued to remain raised, ranging between 29.8 and 76.6 IU/l; αSU levels also remained raised between 2.15 and 5.6 IU/l. Follow‐up MRI scans in 1997, 1999 and 2001 did not show any appreciable changes. Her latest MRI scan in 2003 showed a 7×8‐mm enhancing mass on the right side of the pituitary gland after administration of gadolinium. The optic chiasm and pituitary stalk were normal (fig 1B).
In June 2005, 8 years after her thyroid surgery, the woman was clinically euthyroid on 150 μg thyroxine daily. Serum levels were as follows: FT4, 21.6 pmol/l (normal 10–23 pmol/l); FT3, 4.4 pmol/l (normal 3.5–6.5 pmol/l); TSH, 76.6 mIU/l (normal 0.4–4.5 mIU/l). She was still menstruating, but serum gonadotrophin levels had risen markedly: FSH, 63.5 IU/l; and leutinising hormone, 49.4 IU/l. Oestradiol levels were premenopausal at 71 pmol/l. This suggests that, in addition to TSH, FSH and leutinising hormone were secreted by her pituitary adenoma.
Case 2
A 42‐year‐old man presented in 1982, complaining of palpitations and slight weight loss. After treatment with thyroid drugs for presumed primary hyperthyroidism for 8 years, his serum TSH was inappropriately raised, ranging between 5.6 and 17.3 mIU/l (normal 0.3–5.6 mIU/l), with corresponding FT4 concentrations of 23.7–110.3 pmol/l (normal 9.0–24.5). After discontinuation of carbimazole, investigations showed raised αSU at 1.4 IU/l, raised FSH at 7.9 IU/l, and no marked response of αSU, TSH or gonadotrophins to 200 μg intravenous TSH‐releasing hormone. MRI showed a lobulated mass arising from the right side of the pituitary and extending into the suprasellar cistern. The patient underwent uncomplicated trans‐sphenoidal adenomectomy in 1991, and histopathological analysis confirmed a pituitary adenoma with positive immunostaining for TSH, FSH and growth hormone. Details of his presentation, initial treatment and clinical course up to 6 months after trans‐sphenoidal surgery have been published previously.2 Tissue culture studies supported the clinical diagnosis of a TSH and gonadotrophin‐secreting adenoma.
Six months postoperatively, FT4 remained raised at between 50 and 60 pmol/l, TSH and FSH were still inappropriately high at 1.2 mIU/l and 5.5 IU/l, respectively, but αSU level had fallen within the normal range at 0.4 IU/l. Thyroid autoantibodies were negative. A repeat MRI scan showed no marked residual adenomatous material in the pituitary fossa.
In subsequent months, the patient remained clinically thyrotoxic with inappropriate TSH secretion. He declined further pituitary surgery or irradiation and opted for radioiodine treatment in November 1993. An MRI scan in 1994 showed some residual soft‐tissue material in the pituitary fossa and a small nodule extending into the suprasellar cistern on the right side that enhanced with gadolinium administration. Further MRI scans in 1995 and 1997 (fig 1C) showed no appreciable changes. At review in 1997, the patient was clinically euthyroid; his serum levels were as follows: TSH, 3.5 mIU/l; FT4, 24.3 pmol/l; FT3, 5.6 pmol/l; αSU, 0.4 IU/l; FSH, 4.6 IU/l; leutinising hormone, 3.0 IU/l. Similar clinical and biochemical pictures were seen again in 1999, 2001, 2002, 2003 and 2004. In 2004, an MRI scan showed a 6×5‐mm mass in the right side of the pituitary fossa that enhanced with gadolinium administration (fig 1D).
In October 2005, 12 years after his ablative thyroid treatment and 14 years after his trans‐sphenoidal adenomectomy, the patient was well, clinically euthyroid, and serum levels were as follows: TSH, 3.3 mIU/l; FT4, 21.4 pmol/l; FT3, 4.7 pmol/l; FSH, 3.1 IU/l; leutinising hormone, 3.2 IU/l, αSU, 0.55 IU/l; prolactin, 53 mIU/l.
Discussion
The primary goals of treatment of TSH‐secreting pituitary adenomas (TSHomas) are to remove the tumour and restore euthyroidism. However, in some patients, either one or both of these goals are not achieved. Moreover, in other cases, patients may be assessed as having high surgical risk or may decline to undergo surgery or pituitary irradiation. Other medical treatments will then have to be used to control hyperthyroidism and, if possible, to facilitate tumour mass shrinkage.
The principal medical treatment of TSHomas is administration of long‐acting somatostatin analogues. These have been shown in small, non‐randomised trials to lead to a reduction in TSH secretion, with restoration of the euthyroid state in most cases and pituitary tumour mass shrinkage in about 50% of patients.3,4,5,6,7,8 However, somatostatin analogues fail to restore euthyroidism in as many as 25% of patients, and their use can be limited as a result of their side‐effect profile and their high cost.3,5,9
Medical or surgical treatments directed at the thyroid gland itself have long been regarded with caution in case of aggressive transformation of the tumour as observed in Nelson's syndrome after adrenalectomy for Cushing's disease.1 This argument has been supported by two main observations: firstly, TSH levels in patients previously treated with thyroid ablation have been found to be up to sixfold that in untreated patients1; secondly, in previously treated patients, a more frequent occurrence of invasive macroadenomas has been observed,1 although this was not the case in a series of 25 patients with TSHomas from a single institution.10 It has been postulated that the tumorous thyrotoph cells may retain at least partially their responsiveness to feedback mechanisms by increasing secretion of TSH and by undergoing more active cellular proliferation in response to even small reductions in circulating thyroid hormone levels, as occurs with thyroid ablative treatments.1
Against this assumption is the well‐documented unresponsiveness of TSH‐secreting pituitary adenomas to high‐dose T3, as seen during the T3 suppression test, which describes well the autonomy of pituitary thyrotrophs to thyroid hormone feedback in patients with TSHomas.1
As these tumours are rare, there have been very few opportunities to study in detail their precise biological behaviour and properties. Although some data have confirmed the insensitivity of neoplastic thyrotrophs to thyroid hormone feedback,11,12 data from other studies suggest doubt regarding these findings.13,14,15
Misdiagnosis of TSHomas as primary hyperthyroidism and previous ablative thyroid treatment have often been associated with the presence of invasive macroadenomas presenting with endocrine dysfunction and compressive symptoms such as visual field defects.1 However, the extent to which the treatment itself contributed to tumour progression remains unclear. The number of TSH‐secreting microadenomas misdiagnosed and treated as primary hyperthyroidism that remained confined to the pituitary fossa and never caused optic chiasmal compression or major endocrine abnormalities remains unknown. The lack of these symptoms and signs may have resulted in these tumours being less likely to be referred for more detailed specialist evaluation and investigation, which is how most of the thyrotropinomas reported in the literature had been discovered.
To our knowledge, these are the first two cases of thyrotropinomas where the size and extent of the pituitary adenoma were clearly documented before ablative thyroid treatment was started and where they were subsequently closely monitored over 8 and 12 years. In the first case described, histopathological confirmation of a TSH‐secreting adenoma was not obtained, as the patient declined pituitary surgery. However, the biochemical, radiological and genetic profiles all supported a diagnosis of a TSHoma instead of pituitary resistance to thyroid hormones, which could have been an alternative diagnosis. These two cases show that ablative thyroid treatment can be a safe and successful option for TSHomas in specific situations when other treatments are declined or contraindicated. We have shown that thyroidectomy carried out in a patient with a TSHoma confined to the pituitary fossa does not necessarily lead to aggressive transformation of the microadenoma, and that radioiodine can be successfully given and can restore euthyroidism in patients with residual adenomatous tissue and persistent TSH hypersecretion after trans‐sphenoidal surgery. However, long‐term and close follow‐up of these patients is mandatory to ensure that the size and behaviour of their tumours do not change markedly.
Acknowledgements
We thank Professor Chatterjee and staff at his laboratory, University of Cambridge, Cambridge, UK, who carried out the thyroid hormone receptor β gene analysis for case 1.
Abbreviations
αSU - α‐subunit
FSH - follicle‐stimulating hormone
FT3 - free tri‐iodothyronine
FT4 - free thyroxine
MRI - magnetic resonance imaging
TSH - thyrotropin
TSHoma - TSH‐secreting pituitary adenoma
Footnotes
Competing interests: None declared.
Informed consent was obtained for publication of the patients' details described in this report.
References
- 1.Beck‐Peccoz P, Brucker‐Davis F, Persani L.et al Thyrotropin‐secreting pituitary tumors. Endocr Rev 199617610–638. [DOI] [PubMed] [Google Scholar]
- 2.Patrick A W, Atkin S L, MacKenzie J.et al Hyperthyroidism secondary to a pituitary adenoma secreting TSH, FSH, alpha‐subunit and GH. Clin Endocrinol (Oxford) 199440275–278. [DOI] [PubMed] [Google Scholar]
- 3.Chanson P, Weintraub B D, Harris A G. Octreotide therapy for thyroid‐stimulating hormone‐secreting pituitary adenomas. A follow‐up of 52 patients. Ann Intern Med 1993119236–240. [DOI] [PubMed] [Google Scholar]
- 4.Gancel A, Vuillermet P, Legrand A.et al Effects of a slow‐release formulation of the new somatostatin analogue lanreotide in TSH‐secreting pituitary adenomas. Clin Endocrinol (Oxford) 199440421–428. [DOI] [PubMed] [Google Scholar]
- 5.Comi R J, Gesundheit N, Murray L.et al Response of thyrotropin‐secreting pituitary adenomas to a long‐acting somatostatin analogue. N Engl J Med 198731712–17. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn J M, Arlot S, Lefebvre H.et al Evaluation of the treatment of thyrotropin‐secreting pituitary adenomas with a slow release formulation of the somatostatin analog lanreotide. J Clin Endocrinol Metab 2000851487–1491. [DOI] [PubMed] [Google Scholar]
- 7.Caron P, Arlot S, Bauters C.et al Efficacy of the long‐acting octreotide formulation (octreotide‐LAR) in patients with thyrotropin‐secreting pituitary adenomas. J Clin Endocrinol Metab 2001862849–2853. [DOI] [PubMed] [Google Scholar]
- 8.Taylor T J, Donlon S S, Bale A E.et al Treatment of a thyrotropinoma with octreotide‐LAR in a patient with multiple endocrine neoplasia‐1. Thyroid 2000101001–1007. [DOI] [PubMed] [Google Scholar]
- 9.Beck‐Peccoz P, Mariotti S, Guillausseau P J.et al Treatment of hyperthyroidism due to inappropriate secretion of thyrotropin with the somatostatin analog SMS 201–995. J Clin Endocrinol Metab 198968208–214. [DOI] [PubMed] [Google Scholar]
- 10.Brucker‐Davis F, Oldfield E H, Skarulis M C.et al Thyrotropin‐secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. J Clin Endocrinol Metab 199984476–486. [DOI] [PubMed] [Google Scholar]
- 11.Filetti S, Rapoport B, Aron D C.et al TSH and TSH‐subunit production by human thyrotrophic tumour cells in monolayer culture. Acta Endocrinol (Copenh) 198299224–231. [DOI] [PubMed] [Google Scholar]
- 12.Jaquet P, Hassoun J, Delori P.et al A human pituitary adenoma secreting thyrotropin and prolactin: immunohistochemical, biochemical, and cell culture studies. J Clin Endocrinol Metab 198459817–824. [DOI] [PubMed] [Google Scholar]
- 13.Lamberts S W, Oosterom R, Verleun T.et al Regulation of hormone release by cultured cells from a thyrotropin‐growth hormone‐secreting pituitary tumor. Direct inhibiting effects of 3,5,3′‐triiodothyronine and dexamethasone on thyrotropin secretion. J Endocrinol Invest 19847313–317. [DOI] [PubMed] [Google Scholar]
- 14.Le D M, Brandi A M, Kujas M.et al Thyrotropin‐releasing hormone (TRH) binding sites and thyrotropin response to TRH are regulated by thyroid hormones in human thyrotropic adenomas. Eur J Endocrinol 1994130559–564. [DOI] [PubMed] [Google Scholar]
- 15.Sarapura V D, Wood W M, Gordon D F.et al Effect of thyroid hormone on T3‐receptor mRNA levels and growth of thyrotropic tumors. Mol Cell Endocrinol 19939175–81. [DOI] [PubMed] [Google Scholar]