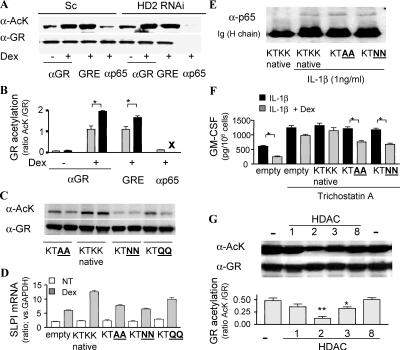
Figure 4.
GR deacetylation by HDAC2 is a prerequisite for p65–NF-κB binding. (A) GR was precipitated after vehicle or Dex treatment with anti-GR antibody in whole cell extracts and with GRE oligonucleotides or anti-p65–NF-κB antibody in the presence of 10−8 M Dex and 1 ng/ml IL-1β. Bands were visualized by anti–acetyl-lysine antibody (α-AcK) and anti-GR antibody (α-GR). (B) Graphical representation of the results shown in A, with the ratio of AcK band to GR band in nontreated (shaded bar) and HDAC2 RNAi cells (closed bar). X represents no GR recruitment to NF-κB. *, P < 0.05. (C) GR acetylation level of each site-directed mutant after treatment with 10−6 M Dex for 1 h. GR were pulled down with anti–His-tag antibody. (D) SLPI mRNA level after treatment with 10−6 M Dex for 4 h. (E) GR binding to p65 under treatment with 10−8 M Dex for 1 h. GR is immunoprecipitated with His-tag antibody 1 h after IL-1β treatment. (F) Effect of 10−8 M Dex on IL-1β–induced GM-CSF production in the presence of 10 nM TSA for 10 min. *, P < 0.05. (G) AcK detection on immunoprecipitated GR in the presence of 10−6 M Dex after incubation with HDAC1, -2, -3, or -8 for 4 h at 30(C. (bottom) The ratio of AcK band to GR band is shown graphically. * and **, P < 0.05 and P < 0.01, respectively, versus control (n = 3 experiments). Values in B, D, F, and G represent means ± SEM.