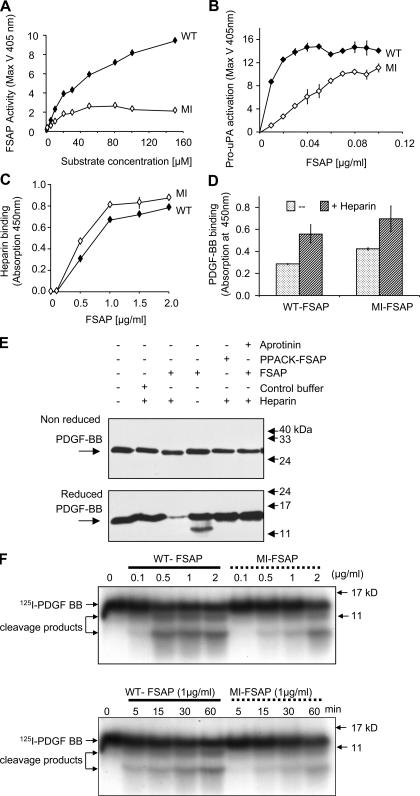
Figure 1.
Enzymatic and binding properties of WT- and MI-FSAP. (A) WT- and MI-FSAP (0.33 μg/ml each) were incubated with increasing concentrations of the chromogenic substrate (H-D-Ile-Pro-Arg-pNA) in the presence of heparin (10 μg/ml), and the maximal velocity (MaxV) was measured in a kinetic plate reader (mean ± SD of triplicate wells). (B) Pro-uPA activation (MaxV) was measured, as described previously (3), in the presence of increasing concentrations of WT- and MI-FSAP in the presence of heparin. (C) Indicated concentrations of FSAP were immobilized, and biotinylated heparin-BSA (0.5 μg/ml) was used as a ligand and its binding was detected with streptavidin-coupled peroxidase (mean ± SD of triplicate wells). (D) PDGF-BB (1 μg/ml) was immobilized, and FSAP (0.5 μg/ml) was used as a ligand in the absence or presence of heparin (10 μg/ml) and its binding was detected with anti-FSAP antibody (mean ± SD of triplicate wells). (E) Mixtures of FSAP (or PPACK-FSAP) (10 μg/ml), buffer control, heparin (10 μg/ml), PDGF-BB (1 μg/ml), and aprotinin (15 μg/ml) were incubated for 1 h at 37°C, and Western blot was performed with an anti–PDGF-BB antibody under reducing or nonreducing conditions. (F) 125I–PDGF-BB was incubated with WT- or MI-FSAP in the presence of heparin, and after SDS-PAGE under reducing conditions autoradiography was performed. The effect of different concentrations of FSAP was examined at the 60-min time point (top), and the time course (bottom) was analyzed at FSAP concentration of 1 μg/ml.