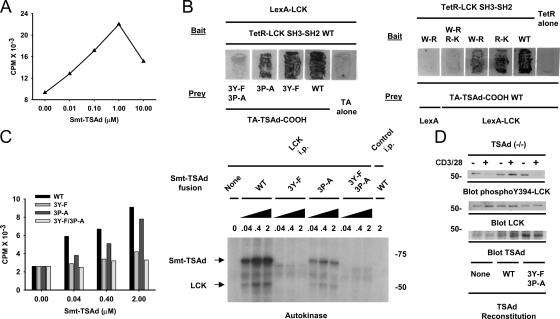
Figure 4.
TSAd activation of LCK kinase activity. (A) LCK was immunoprecipitated from TSAd-deficient LN T cells and tested for its ability to transfer 32P to a SRC peptide substrate in the presence of a tyrosine-phosphorylated His6-Smt3–tagged TSAd fusion protein. (B) Yeast were transformed with the indicated TetR-fused LCK SH3-SH2 bait proteins and TA-fused TSAd COOH region prey proteins. WT, wild-type bait or prey proteins; 3Y-F and 3P-A, Y275F/Y292F/Y317F and P262A/P263A/P265A triple mutants of the TSAd COOH region, respectively; W-R, inactivating W97R mutant of the LCK SH3 domain; R-K, inactivating R154K mutant of the LCK SH2 domain. Yeast were also transformed with the kinase domain of LCK fused to LexA to phosphorylate the TSAd COOH region. The LexA moiety is irrelevant to the system and the kinase domain fusion protein is predicted to diffuse freely within the cell. Growth of yeast on Uracil dropout plates was assessed after 48 h of incubation and taken as an indicator of bait–prey interaction. Note that mutation of tyrosine residues and the proline-rich stretch of the TSAd COOH region are necessary to prevent interaction with LCK. Note also that mutation of the SH2 and SH3 domains of LCK is necessary to prevent interaction with TSAd when the latter is phosphorylated (by LCK). (C) Experiments were conducted as in A using the indicated TSAd fusion proteins. SRC-peptide phosphorylation (left) and autokinase activity (right) was determined. (D) Splenic T cells from TSAd (−/−) mice were transfected with wild-type, 3Y-F/3P-A TSAd, or vector alone (None) and stimulated with optimal concentrations of CD3 and CD28 mAb for 2 min. Phosphorylation of LCK on Y394 was assessed by Western blotting of whole cell lysates using a phospho-specific antibody. Blots were reprobed with LCK and TSAd antibodies to show equivalent loading of LCK and expression of TSAd, respectively. TSAd is observed as a triplet of three proteins of very similar molecular weights in these experiments. The species of intermediate molecular weight comigrates with a nonspecific band that can be identified in T cells transfected with vector alone.