Abstract
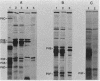
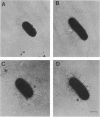
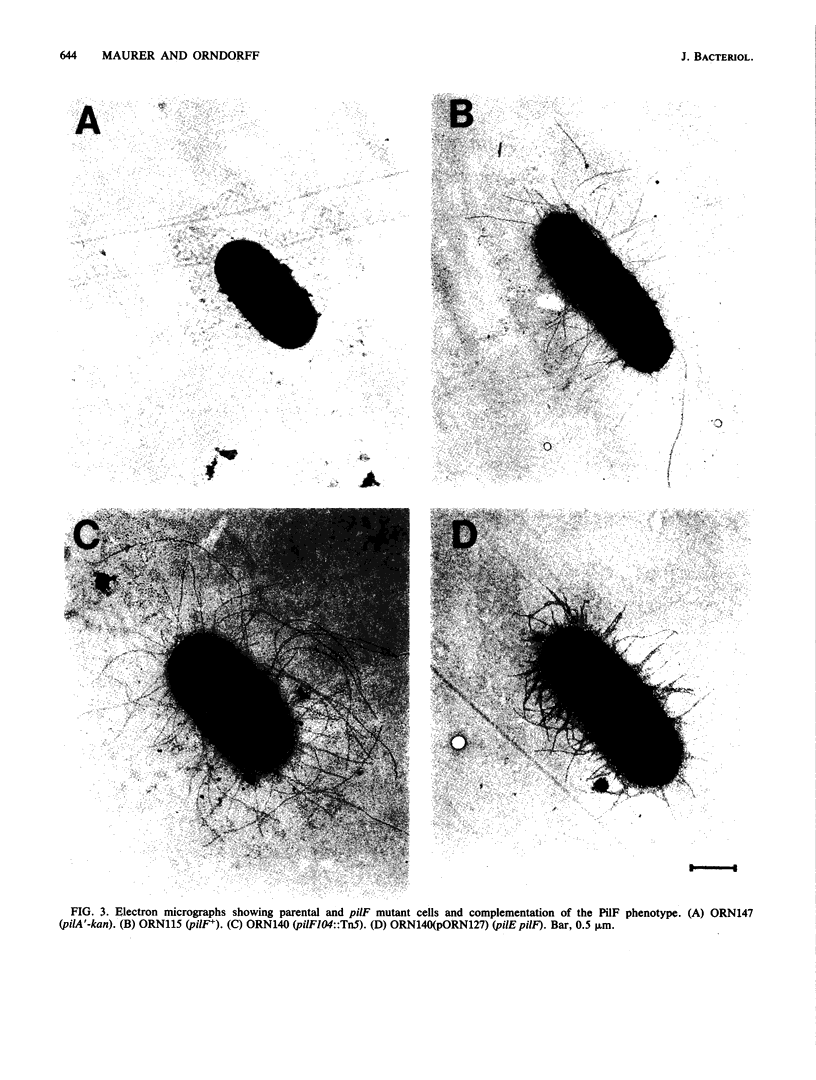
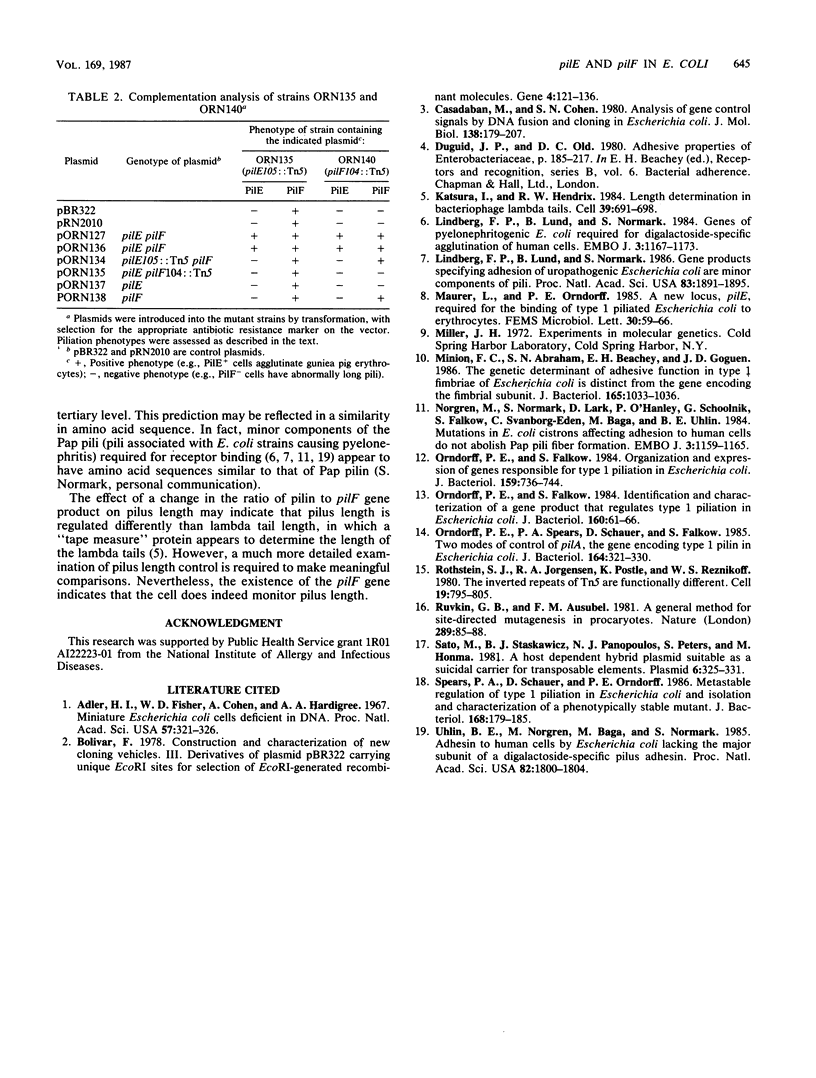
We describe the identification and characterization of two genes and their gene products responsible for determining receptor binding and pilus length in type 1-piliated Escherichia coli. One gene, pilE, conferred the ability of piliated cells to agglutinate guinea pig erythrocytes. The other gene, pilF, determined pilus length, in that mutants having lesions in pilF had very long pili. The two genes were detected after Tn5 mutagenesis of a cloned segment of DNA that normally complemented a pilE lesion in the chromosome. Thus, lesions in pilE or pilF on the cloned segment resulted in mutants having the PilE- phenotype (piliated but unable to agglutinate erythrocytes). Introduction of the plasmid-encoded mutant alleles of pilE and pilF into the chromosome followed by electron microscopic examination of the mutants showed that only lesions in pilF conferred the striking increase in pilus length. Mutations in pilF could be complemented in trans by the original cloned segment to produce cells with parental-length pili. Minicell transcription and translation of the cloned pilE and pilF genes having representative Tn5 insertion mutations showed that the pilE gene product was a protein of ca. 31 kilodaltons and that the pilF gene product was a protein of ca. 18 kilodaltons. We believe that the pilF gene product may act as a competitive inhibitor of pilus polymerization. Thus, pilus length may be controlled by the ratio of pilin to pilF gene product present within the cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Katsura I., Hendrix R. W. Length determination in bacteriophage lambda tails. Cell. 1984 Dec;39(3 Pt 2):691–698. doi: 10.1016/0092-8674(84)90476-8. [DOI] [PubMed] [Google Scholar]
- Lindberg F. P., Lund B., Normark S. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 1984 May;3(5):1167–1173. doi: 10.1002/j.1460-2075.1984.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Normark S. Gene products specifying adhesion of uropathogenic Escherichia coli are minor components of pili. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1891–1895. doi: 10.1073/pnas.83.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minion F. C., Abraham S. N., Beachey E. H., Goguen J. D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J Bacteriol. 1986 Mar;165(3):1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Identification and characterization of a gene product that regulates type 1 piliation in Escherichia coli. J Bacteriol. 1984 Oct;160(1):61–66. doi: 10.1128/jb.160.1.61-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Spears P. A., Schauer D., Falkow S. Two modes of control of pilA, the gene encoding type 1 pilin in Escherichia coli. J Bacteriol. 1985 Oct;164(1):321–330. doi: 10.1128/jb.164.1.321-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Sato M., Staskawicz B. J., Panopoulos N. J., Peters S., Honma M. A host-dependent hybrid plasmid suitable as a suicidal carrier for transposable elements. Plasmid. 1981 Nov;6(3):325–331. doi: 10.1016/0147-619x(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Spears P. A., Schauer D., Orndorff P. E. Metastable regulation of type 1 piliation in Escherichia coli and isolation and characterization of a phenotypically stable mutant. J Bacteriol. 1986 Oct;168(1):179–185. doi: 10.1128/jb.168.1.179-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]