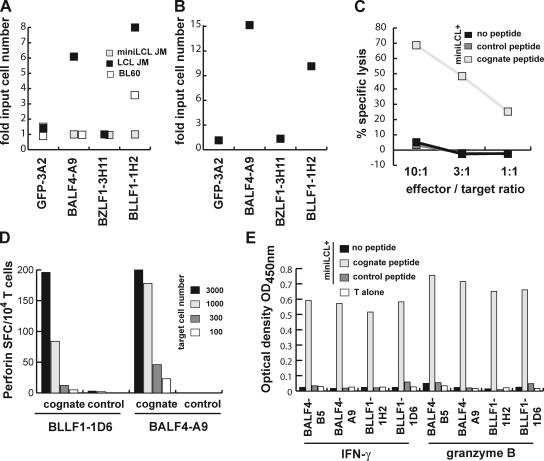
Figure 7.
Glycoprotein-specific CD4+ T cells are cytolytic and inhibit outgrowth and proliferation of LCL. (A) Decreasing numbers of LCL JM, miniLCL JM, or BL60 cells were either cultured alone or cocultured with BLLF1-1H2, BALF4-A9, BZLF1-3H11, or GFP-specific 3A2 T cells (10,000/well). Proliferation of the cells was followed over time and the minimum number of cells proliferating was determined after 4 wk. Coculture of LCL and BL60 cells, but not miniLCL, with glycoprotein-specific T cells increased the minimum number of cells necessary for proliferation 3–10-fold. (B) Purified B cells were infected with EBV and increasing cell numbers plated together with BLLF1-, BALF4-, BZLF1-, or irrelevant GFP-specific T cells (10,000/well). The minimum number of EBV-infected B cells required for LCL outgrowth was ∼10-fold higher in the presence of glycoprotein-specific T cells. (C) MiniLCL JM pulsed with the indicated peptides were cocultured with the BALF4-B5 T cells at different effector to target ratios. Upon antigen recognition, the glycoprotein-specific T cells efficiently lysed the target cells. (D) Serial dilutions of miniLCL JM pulsed with cognate or control peptides were cocultured with glycoprotein-specific T cells, and perforin secretion by the T cells was assayed by ELISPOT. (E) In addition to IFN-γ, all glycoprotein-specific T cell clones also secreted granzyme B in response to target cell recognition.