Abstract
Tumor-associated macrophages are a prominent component of ovarian cancer stroma and contribute to tumor progression. B7-H4 is a recently identified B7 family molecule. We show that primary ovarian tumor cells express intracellular B7-H4, whereas a fraction of tumor macrophages expresses surface B7-H4. B7-H4+ tumor macrophages, but not primary ovarian tumor cells, suppress tumor-associated antigen-specific T cell immunity. Blocking B7-H4-, but not arginase-, inducible nitric oxide synthase or B7-H1 restored the T cell stimulating capacity of the macrophages and contributes to tumor regression in vivo. Interleukin (IL)-6 and IL-10 are found in high concentrations in the tumor microenvironment. These cytokines stimulate macrophage B7-H4 expression. In contrast, granulocyte/macrophage colony-stimulating factor and IL-4, which are limited in the tumor microenvironment, inhibit B7-H4 expression. Ectopic expression of B7-H4 makes normal macrophages suppressive. Thus, B7-H4+ tumor macrophages constitute a novel suppressor cell population in ovarian cancer. B7-H4 expression represents a critical checkpoint in determining host responses to dysfunctional cytokines in ovarian cancer. Blocking B7-H4 or depleting B7-H4+ tumor macrophages may represent novel strategies to enhance T cell tumor immunity in cancer.
Tumor stroma is required for the survival, growth, and progression of cancers. The major components in tumor stroma are fibroblasts, neovasculature, and tumor-associated macrophages. Stroma inhibits infiltration and activation of tumor T cells (1–4). However, it is poorly understood how each cellular component in tumor stroma contributes to tumor immunopathogenesis (5). Mouse tumor macrophages are proposed to subvert tumor-specific immunity. However, the suppressive roles and mechanisms are not well understood in the immunopathogenesis of human cancers.
APCs are critical for initiating and maintaining tumor-associated antigen (TAA)-specific T cell immunity. Tumor macrophages markedly outnumber other APCs, such as DCs, and represent an abundant population of APCs in solid tumors (6–9). Strikingly, numerous studies have investigated the phenotypes and functions of DCs in tumor immunity (5, 10–18). Studies in mice have also revealed that tumor macrophages promote tumor growth and metastasis by directly acting on tumor cells (6–9). Immunohistochemical assessment of the number and the distribution of tumor macrophages in human tumors has yielded scant, and often contradictory, results regarding any potential role in tumor pathogenesis (19–21). Thus, immune functional data are essential for understanding the roles and potential suppressive mechanisms of human tumor environmental macrophages in tumor immunopathogenesis.
B7-H4 (B7x, B7S1), a recently discovered member of the B7 family of T cell costimulatory molecules, is a negative regulator of T cell responses in vitro by inhibiting T cell proliferation, cell cycle progression, and cytokine production (22–25). Antigen-specific T cell responses are impaired in mice treated with a B7-H4Ig fusion protein (22). However, the expression, regulation, and function of B7-H4 are virtually unknown in the context of human immunity.
We recently described several mechanisms in the human ovarian cancer microenvironment that actively defeat tumor immunity (5, 26, 27), including an immunopathologic role for regulatory T cells (26–28). As tumor macrophages are far more abundant than regulatory T cells in the highly dysfunctional ovarian cancer microenvironment, we investigated the possibility that B7-H4+ tumor macrophages are a novel regulatory cell population in ovarian cancer, and also investigated a role for B7-H4 signals in this regard.
RESULTS
Ovarian tumors express intracellular B7-H4
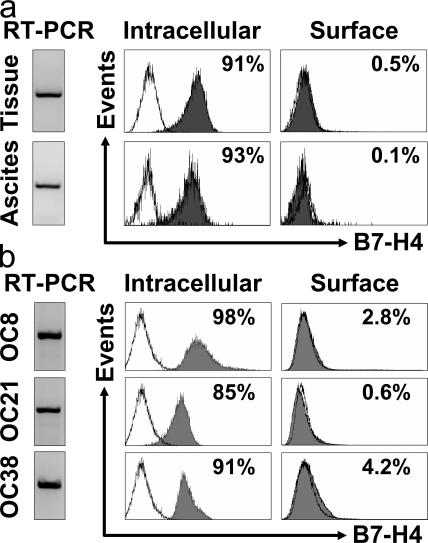
Based on the phenotype of lin−EpCam+CD45−CD14−, we initially isolated and sorted primary fresh ovarian tumor cells from ovarian cancer ascites fluid and from the tumor mass. We observed that fresh ovarian tumors expressed B7-H4 mRNA, and exclusively expressed intracellular rather than cell surface B7-H4 protein (Fig. 1 a). In support of this, our established ovarian tumor cell lines expressed B7-H4 mRNA (Fig. 1 b), but <5% expressed surface B7-H4 and >85% expressed intracellular B7-H4 (Fig. 1 b).
Figure 1.
Ovarian tumor cells express intracellular B7-H4. B7-H4 expression was analyzed by RT-PCR and FACS in (a) fresh primary ovarian tumor cells and (b) ovarian tumor cell lines. FACS results expressed the mean percentage of B7-H4–expressing cells. OC8, OC21, and OC38 are three established ovarian tumor cell lines. Fresh primary ovarian tumor cells were isolated and sorted from ascites and tumor tissues with a phenotype of lin−EpCam+CD45−CD14−. Two of six representative patient samples are shown for panel a. Three of eight cell lines are shown for panel b. Filled histogram, B7-H4 expression; open histogram, isotype control.
Ovarian tumor-associated macrophages express surface B7-H4
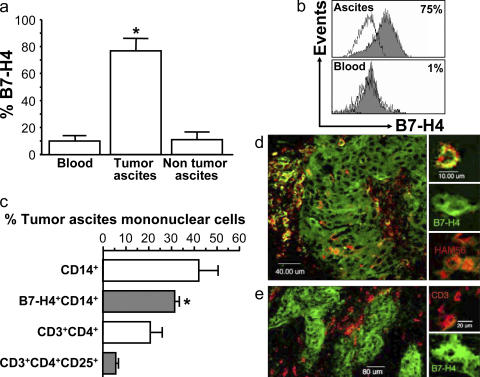
We then studied ovarian tumor macrophages. >70% of freshly isolated tumor macrophages expressed cell surface B7-H4 protein (Fig. 2). Freshly isolated tumor ascites CD14+ tumor macrophages from previously untreated patients with ovarian cancer (n = 12) expressed significantly more B7-H4 compared with macrophages from nonmalignant ascites (liver transplant patients, n = 5) or normal peripheral blood monocytes (n = 25; P = 0.02 for both) (Fig. 2, a and b). The prevalence of B7-H4+ tumor macrophages was markedly higher than that of CD4+CD25+CD3+ regulatory T cells (T reg cells) (n = 12; *, P < 0.001) in tumor ascites (Fig. 2 c). Using confocal microscopy, we confirmed that 70% ± 12% of Ham56+ tumor macrophages (n = 60) (Fig. 2 d) and 100% ovarian epithelial carcinoma cells strongly expressed B7-H4 (Fig. 2 e), whereas tumor-infiltrating CD3+ T cells (Fig. 2 e) and tumor ascites CD3+ T cells did not (unpublished data). Similar levels of B7-H4 expression were observed on CD68+ tumor macrophages. Interestingly, tumor islets were generally surrounded by B7-H4+ tumor macrophages (Fig. 2, d and e). Together, these data indicate selective cell surface expression of B7-H4 on tumor macrophages.
Figure 2.
Tumor-associated macrophages express B7-H4. (a and b) Tumor ascites macrophages express B7-H4. FACS analysis showed that ascites macrophages, but not control nonmalignant ascites macrophages, or fresh normal blood monocytes expressed B7-H4. Results are expressed as the mean of the percentage of B7-H4+ cells ± SEM in total macrophages. Filled histogram, B7-H4 staining; open histogram, isotype control. (c) High prevalence of B7-H4+ tumor macrophages in tumor ascites. FACS analysis was used to determine the prevalence of B7-H4+ tumor macrophages and CD3+CD4+CD25+ cells in tumor ascites (*, P < 0.001). Results are expressed as the mean of percentage ± SEM in tumor ascites mononuclear cells. (d and e) Tumor tissues were stained with anti–human B7-H4, anti–human CD3, anti–human-Ham56, and control antibody as described in Materials and methods, and analyzed with confocal microscope. (d) Tumor mass macrophages and tumor cells express B7-H4. B7-H4, green; Ham56, red. Tumor macrophages are identified as Ham56+ cells (red). Ham56+B7-H4+ cells are yellowish. Large numbers of tumor macrophages form a barrier surrounding tumor islets. (e) Tumor-infiltrating T cells are B7-H4−. Tumor cells, but not CD3+ T cells (red), expressed B7-H4 (green). 1 of 60 representative patient samples is shown for panels d and e.
Phenotypes of B7-H4+ tumor macrophages
To study the nature of B7-H4+ tumor macrophages, we compared the phenotypes of B7-H4+ tumor macrophages with B7-H4− tumor macrophages. Most of the T cell activation-related molecules (HLA-DR, HLA-ABC, CD40, CD54, and CD80) were expressed at similar levels on B7-H4+ tumor macrophages and B7-H4− tumor macrophages (Table S1, available at http://www.jem.org/cgi/content/full/jem.20050930/DC1). However, B7-H4+ tumor macrophages expressed higher levels of CD86 than B7-H4− tumor macrophages (P = 0.04) (Table S1). Furthermore, after LPS stimulation both B7-H4+ tumor macrophages and B7-H4− tumor macrophages produced comparable levels of several cytokines including IL-6, IL-10, and TNF-α (unpublished data).
Tumor microenvironmental factors induce macrophage B7-H4 expression
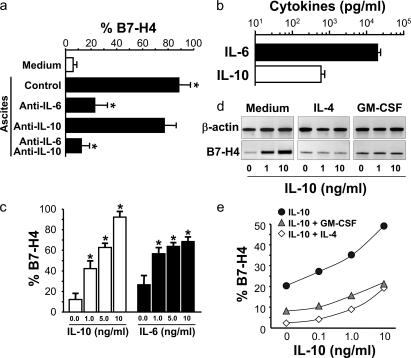
Because tumor macrophages, but not normal macrophages or monocytes expressed high level B7-H4, we hypothesized that tumor microenvironmental factors may stimulate B7-H4 expression on tumor macrophages. To test this, we incubated normal blood monocytes with serum-free medium or tumor ascites. Tumor ascites induced significant B7-H4 expression (n = 12; P < 0.0001) (Fig. 3 a). Addition of a neutralizing monoclonal antibody against IL-6 significantly blocked tumor ascites-mediated B7-H4 up-regulation (n = 5; P < 0.001) (Fig. 3 a), and neutralizing monoclonal antibody against IL-10 partially reduced tumor ascites-mediated B7-H4 up-regulation (n = 5; P = 0.053) (Fig. 3 a). Simultaneous blockade of IL-6 and IL-10 completely abrogated tumor ascites-induced B7-H4 (Fig. 3 a), suggesting that IL-6 and IL-10 in tumor ascites cooperate to stimulate macrophage B7-H4 expression. In support, we detected high levels of IL-6 and IL-10 mRNA in ovarian tumor tissues (unpublished data) and protein in tumor ascites fluid (n = 11) (Fig. 3 b). We further incubated ovarian tumor cells with tumor ascites. Tumor ascites had no effect on intracellular or cell surface B7-H4 expression in tumor cells (Fig. S1 A, http://www.jem.org/cgi/content/full/jem.20050930/DC1).
Figure 3.
Tumor ascites IL-6 and IL-10 stimulate macrophage B7-H4 expression. (a) Tumor ascites stimulated macrophage B7-H4 expression. Fresh normal blood monocytes were cultured 72 h with ovarian tumor ascites in the presence or absence of anti–human IL-6 or anti–human IL-10 antibodies. B7-H4 expression was analyzed by FACS. Results are expressed as the mean of B7-H4+ cell percentage ± SEM in total macrophages. (b) High concentrations of IL-6 and IL-10 in tumor ascites were detected by ELISA. (c) Recombinant IL-6 or IL-10 stimulated macrophage B7-H4 protein in a dose-dependent manner after a 72 h culture. B7-H4 expression was analyzed by FACS. Results are expressed as the mean of B7-H4+ cell percentage ± SEM in total macrophages. (d and e) Recombinant IL-4 and GM-CSF reduced macrophage B7-H4 mRNA and protein induction. Fresh blood monocytes were cultured with different concentrations of cytokines. (d) B7-H4 mRNA was detected by RT-PCR at 24 h. (e) B7-H4 surface protein was detected by FACS at 72 h. One of seven independent experiments is shown.
Tumor environmental IL-6 and IL-10 induce macrophage B7-H4 expression
In further support of these data, recombinant IL-6 and IL-10 stimulated significant B7-H4 protein expression on normal blood monocytes in a dose-dependent manner (n = 12; P < 0.05 for all) (Fig. 3 c). In further support, recombinant IL-10 induced a dose-dependent induction of B7-H4 mRNA expression (Fig. 3 d). High levels of vascular endothelial growth factor (VEGF) and stromal-derived factor (SDF)-1 were found in ovarian tumor environments (11, 29). We observed that VEGF and SDF-1 had no effects on monocyte B7-H4 expression (unpublished data). Interestingly, IL-4 and GM- CSF blocked the IL-10–induced B7-H4 expression of mRNA (Fig. 3 d) and protein (Fig. 3 e) (n = 7; P < 0.05 for all, compared with medium). Finally, negligible levels of IL-4 and GM-CSF were detected in tumor ascites (<10 ng/ml for each) and tumor tissues (unpublished data). These data indicate that an imbalanced tumor environmental cytokine pattern (high IL-10 and IL-6, low IL-4 and GM-CSF) may condition local macrophages to become dysfunctional, and that stimulation of B7-H4 might be a potential mechanism. As expected, recombinant IL-6, IL-10, IL-4, and GM-CSF had no effect on B7-H4 mRNA and protein expression in tumor cells (Fig. S1, b and c). The data suggest a distinct regulatory mechanism for B7-H4 expression on tumor cells compared with APCs.
B7-H4+ tumor macrophages suppress Her-2/neu-specific T cell immunity
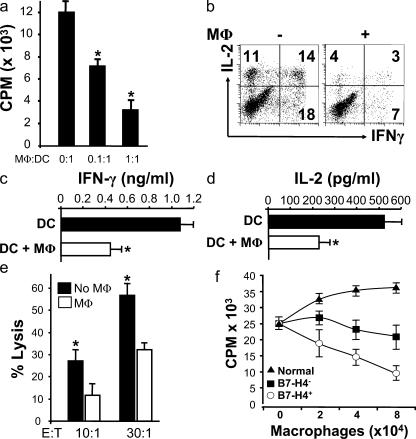
To determine the immunopathological significance of tumor macrophages, we used our in vitro TAA-specific culture system (10, 11). Myeloid dendritic cells (MDCs) were differentiated from HLA-A2+ monocytes as we described previously (30), and loaded with HLA-A2-specific, Her-2/neu peptides (TAA-MDC) (26, 31). Addition of autologous TAA-MDC induced specific T cell activation as expected (Fig. 4). Autologous tumor macrophages isolated from tumor tissues of HLA A2+ patients with Her-2/neu+ tumors significantly inhibited MDC-mediated, Her2/neu-specific T cell proliferation in a dose-dependent manner (n = 5; P < 0.05) (Fig. 4 a). IFN-γ (Fig. 4, b and c) and IL-2 (Fig. 4, b and d) production (n = 5; *, P < 0.01) and Her-2/neu–specific cytotoxicity (n = 5; P < 0.05) (Fig. 4 e) were also inhibited by addition of autologous tumor macrophages. Tumor macrophages isolated from tumor ascites showed similar suppressive effects. MDCs alone did not induce T cell activation (<2,000 counts per minute [cpm]). Limited T cell proliferation was observed in the absence of TAA-MDC stimulation (<1,000 cpm). Further, when tumor macrophages were replaced with irradiated autologous ovarian tumor cells in the identical experimental setting, irradiated autologous tumor cells had no effects on T cell activation (unpublished data).
Figure 4.
Tumor macrophages suppress TAA-specific T cell immunity in vitro. (a–e) Autologous tumor ascites CD3+CD25− T cells were stimulated with Her-2/neu peptide-loaded MDC (TAA-MDC) as described in Materials and methods. (a) Tumor T cell proliferation was detected by [3H]thymidine incorporation (CPM). Results are expressed as the mean of cpm ± SEM. TAA-specific T cell IFN-γ (b and c) and IL-2 (b and d) production was detected by intracellular staining (FACS) and ELISA. (e) Tumor macrophages inhibited Her-2/neu–specific T cell cytotoxicity. MDC-activated Her-2/neu–specific T cells were effector cells, and Her-2/neu peptide-loaded T2 cells were target cells. Her-2/neu–specific cytotoxicity was determined by FACS (see Materials and methods). Tumor macrophage to TAA-MDC ratio was 1:1 for panels b–e. (f) B7-H4+ tumor macrophages significantly suppress T cell proliferation. B7-H4+ or B7-H4− tumor macrophages were sorted from malignant ascites using a FACSaria. Normal macrophages were from M-CSF–treated normal peripheral blood monocytes. Tumor ascites CD3+ T cells were stimulated with anti-CD3 antibody and blood monocytes for 3 d in the presence of different concentrations of tumor macrophages or normal macrophages. T cell proliferation was detected by [3H]thymidine incorporation. Results are expressed as the mean of cpm ± SEM. TAM, tumor macrophages; macrophage, MΦ.
We then sorted B7-H4+ and B7-H4− tumor macrophages to define their functional characteristics. Normal peripheral blood monocytes were cultured for 48 h with M-CSF to generate normal macrophages (26). Normal macrophages induced dose-dependent T cell proliferation (Fig. 4 f). However, both B7-H4+ and B7-H4− tumor macrophages significantly suppressed T cell proliferation in a dose-dependent manner (n = 5; P < 0.01 for all, compared with normal macrophages and no macrophages) (Fig. 4 f). Furthermore, B7-H4+ tumor macrophages were two- to sixfold more potent than B7-H4− tumor macrophages in mediating T cell suppression (n = 5; P < 0.01 for all, compared with normal macrophages and no macrophages) (Fig. 4 f). Further, >70% tumor macrophages express B7-H4 (Fig. 2). These data suggest that total tumor macrophage-mediated immunosuppression comes predominantly from the B7-H4+ tumor macrophage subset.
Role of B7-H4 on macrophages mediates immunosuppression in vitro
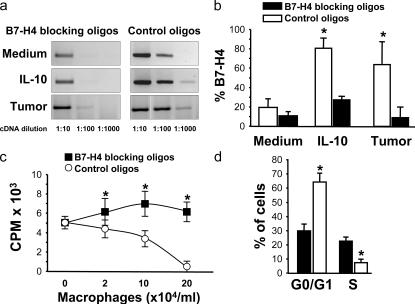
We then focused on B7-H4+ tumor macrophages to determine their role in T cell suppression. As blocking B7-H4 antibody is not available, we designed B7-H4–specific morpholino antisense oligonucleotides (B7-H4 blocking oligos) and an inverted control oligonucleotide (control oligos). We initially studied the effect of medium, IL-10, and tumor ascites-conditioned macrophages. The B7-H4–blocking oligos, but not control oligos, significantly inhibited basal, IL-10, and tumor ascites-induced B7-H4 mRNA expression by over 1,000-fold (Fig. 5 a) and B7-H4 protein expression by over 3-fold (n = 6; P < 0.05) (Fig. 5 b). Neither B7-H4–blocking oligos nor control oligos affected macrophage CD14, CD40, CD80, CD86, B7-H1, HLA-ABC, HLA-DR, or IL-6 and TNF-α expression (unpublished data). These data indicate that our B7-H4–blocking oligos selectively block B7-H4 expression in macrophages.
Figure 5.
Blockade of B7-H4 improved macrophage-mediated T cell activation. (a and b) B7-H4–blocking oligos reduced macrophage B7-H4 expression. Fresh blood monocytes were exposed to B7-H4–blocking oligos and control oligos with medium, tumor ascites, and IL-10. (a) B7-H4 mRNA was detected at 24 h by RT-PCR with serial dilutions of cDNA. (b) B7-H4 protein was detected at 72 h by FACS. One of nine independent experiments is shown. (c and d) Blockade of B7-H4 improved tumor ascites-conditioned macrophage-mediated T cell activation in vitro. Tumor ascites-conditioned macrophages were previously treated with B7-H4–blocking oligos or control oligos and were subsequently subject to activating T cells for 72 h. (c) T cell proliferation was measured by [3H]thymidine incorporation. Results are expressed as the mean of cpm ± SEM. (d) Cell cycle in T cells was analyzed by FACS. Results are expressed as the mean of the percentage of T cells ± SEM in each cycle phase. The ratio between macrophages and T cells was 1:1 for panel d. n = 4–8; *, P < 0.05 compared with control oligos.
We further tested whether B7-H4–blocking oligos affected tumor ascites-conditioned macrophage function. T cell proliferation was significantly increased when stimulated with tumor ascites-conditioned macrophages initially exposed to B7-H4–blocking oligos compared with control oligos (n = 4; P < 0.001) (Fig. 5 c). This coincided with a significant decrease in B7-H4 expression (Fig. 5, a and b). Further, B7-H4–blocking oligos promoted T cell exit from G0/G1 phase and entry into S phase (n = 3; P < 0.05) (Fig. 5 d). Together, these data indicate that B7-H4 contributes to tumor macrophage-mediated immunosuppression in vitro.
B7-H4+ macrophages mediate an immunosuppression independent of B7-H1, arginase and inducible nitric oxide synthase (iNOS)
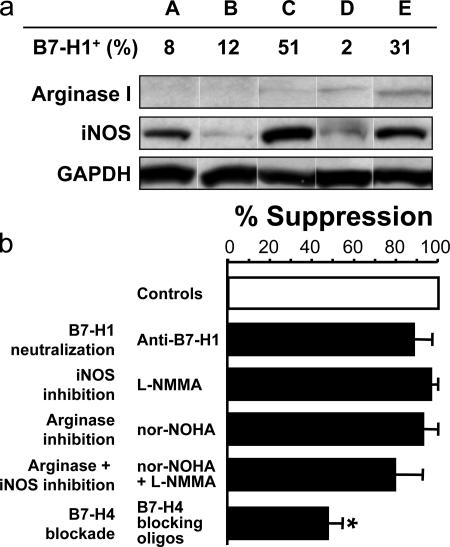
B7-H1, arginase, and iNOS were reported to be implicated in dendritic cells, mouse macrophage, and myeloid suppressor cell–mediated T cell suppression (32–41). To study the additional suppressive mechanism of tumor macrophages, we first analyzed the expression of B7-H1, arginase, and iNOS in B7-H4+ macrophages from ovarian cancer ascites. Flow cytometry analysis demonstrated that the levels of B7-H1 expression were significantly low (20% in average, P < 0.05) and variable (from 2 to 51%) on B7-H4+ macrophages (Fig. 6 a). Western blot analysis revealed limited arginase expression and variable iNOS expression in macrophages among patients (Fig. 6 a). We further tested the role of B7-H1, arginase, and iNOS in T cell activation. We observed that blocking B7-H1 with specific B7-H1–neutralizing antibody (anti–B7-H1) (10, 42, 43), the addition of selective inhibitors of iNOS (L-NMMA) and of arginase (nor-LOHA) (32–41) slightly (<13%), but not significantly, reduced macrophage-mediated T cell suppression compared with treatment with isotype-matched control antibody and control oligos (Fig. 6 b). As expected, blocking B7-H4 with B7-H4–specific oligos significantly reduced the suppression (n = 5; *, P < 0.05) (Fig. 6 b). The data indicate that B7-H4, but not B7-H1, arginase, and iNOS, play a predominant role in B7-H4+ macrophage-mediated suppression.
Figure 6.
B7-H4+ macrophages mediate an immunosuppression independent of B7-H1, arginase, and iNOS. (a) B7-H1, arginase, and iNOS expression on tumor ascites-associated macrophages. B7-H1 expression was analyzed by FACS. Arginase I and iNOS expression was detected by Western blot. A, B, C, D, and E represent 5 individual patients with ovarian cancer. (b) Tumor ascites-conditioned macrophages mediated T cell suppression through B7-H4. T cells were stimulated with tumor ascites-conditioned macrophages for 72 h in the presence of the indicated conditions. T cell proliferation was measured by [3H]thymidine incorporation in the last 16 h. The results were expressed as the percentage of suppression (mean ± SEM). The ratio between macrophages and T cells was 1:2 for panel d. n = 5; *, P < 0.05.
Ectopic B7-H4 expression confers macrophage suppressive capacity
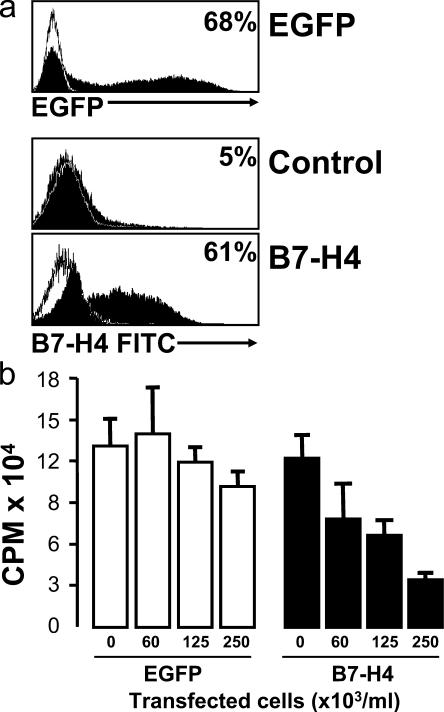
We tested whether B7-H4 expression was sufficient to confer a suppressive phenotype on B7-H4–negative normal cells by transducing normal blood monocytes with human B7-H4 cDNA. 20 h after transfection, 50–70% cells expressed B7-H4 or GFP (positive control), whereas <5% B7-H4 expression was observed on cells without transfection (negative control) (Fig. 7 a). We observed similar transfection efficiency with B7-H1 transfection (unpublished data). B7-H4–transfected cells were subject to coculture with T cells. As expected, B7-H4–transfected cells, but not control-transfected cells or nontransfected cells, suppressed T cell proliferation in a dose-dependent manner (n = 4; P < 0.05 for all, compared with controls) (Fig. 7 b). These data indicate that ectopic B7-H4 expression confers macrophage suppressive capacity.
Figure 7.
Forced B7-H4 expression confers suppressive capacity of normal macrophages. Fresh blood monocytes were transfected using a plasmid encoding human B7-H4 or EGFP cDNA or control plasmid carried the reversed cDNA sequence of B7-H4. (a) 20 h after transfection, B7-H4 and GFP expression were detected by FACS. One of five experiments is shown. (b) Different concentrations of the transfected cells were added into T cell culture in the presence of anti–human CD3 and normal monocytes. T cell proliferation was detected by [3H]thymidine incorporation on day 3. One of five experiments is shown.
Blocking B7-H4 on macrophages improves TAA-specific T cell immunity in vivo
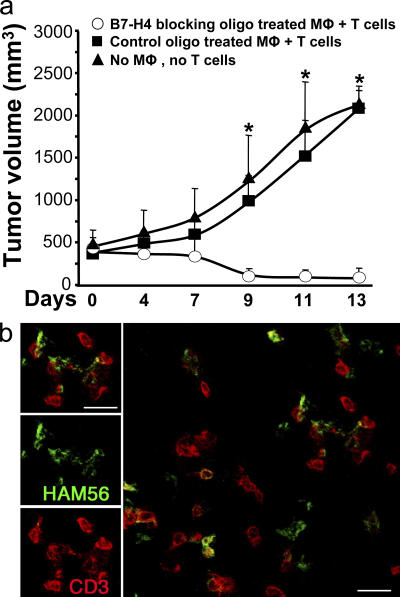
To test the effect of macrophage B7-H4 on TAA-specific T cell immunity in vivo, we treated tumor ascites-conditioned macrophages with B7-H4–blocking oligos or control oligos and then injected them plus autologous TAA-specific T cells into our established human NOD/SCID mice bearing the corresponding ovarian tumors (26). As expected, mice without T cell transfusion, and mice treated with TAA-specific T cells plus control oligo-treated macrophages, showed progressive tumor growth. Supporting our hypothesis that tumor macrophage B7-H4 signals are immunopathologic in vivo, mice treated with TAA-specific T cells plus B7-H4–blocking oligo-treated macrophages showed reduced tumor volumes at each time point from day 7 (n = 5–7 mice per group; *, P < 0.05, compared with B7-H4–blocking oligo) (Fig. 8 a). These data indicate that tumor macrophages defeat TAA-specific T cell immunity in vivo through B7-H4 signals and contribute to tumor growth. Further, confocal microscopic analysis showed that macrophages and T cells were localized in the tumors and the engagement between macrophages and T cells were found in the tumor (Fig. 8 b).
Figure 8.
Blockade of B7-H4 improved macrophage-mediated T cell activation in vivo. (a) Blocking tumor ascites-conditioned macrophage B7-H4 results in tumor regression in vivo. Mice were injected with human primary ovarian tumors as described in Materials and methods. Controls received no additional injection (▴, group 1). Treatments were tumor-specific T cells plus B7-H4–blocking oligo-treated macrophages (◯, group 2), and tumor-specific T cells plus control oligo-treated macrophages (▪, group 3). Mean ± SD of tumor volumes is shown (n = 5–7 mice per group). The T cell injection day was counted as day 0. Macrophage, MΦ. (b) Macrophages and T cell were colocalized in tumor tissue. Similar results were observed in group 2 and 3. Macrophages were identified as Ham56+ cells (green) and T cells as CD3+ cells (red). n = 5.
DISCUSSION
After several decades of neglect, immune suppressor cells are once again an area of active research interest. The best studied immune suppressor population is the well-known yet still relatively newly described CD4+CD25+ T reg cells (44–46). Human T reg cells (26, 47) disable TAA-specific T cell immunity (5, 27). We now report a novel suppressive cell population in patients with ovarian carcinoma, namely B7-H4+ macrophages.
Based on studies in murine systems, macrophages are functionally classified into M1 and M2 (48). Functionally, M1s may produce IL-12, TNF-α, and nitric oxide and induce T helper 1 responses, whereas M2s may produce arginase, but not IL-12, and play an important role in tissue repair and development. Analogously, in the context of tumor immunity, M1s were suggested to induce protective tumor immunity and result in tumor regression, whereas M2s were suggested to promote tumor growth and tumor angiogenesis (6, 7, 32, 34, 48). For example, studies in murine tumor models demonstrate that myeloid suppressor cells, including immature macrophages, suppress T cell activation by various mechanisms including releasing NO and arginase (32–41, 49). Interestingly, a recent study demonstrated that human prostate cancer cells apply similar mechanisms to induce T cell unresponsiveness (39, 40). Strikingly, immunohistochemical assessment of the number and the distribution of tumor macrophages in human tumors has yielded scant and often contradictory results regarding any potential role in tumor immunopathogenesis (19–21). To address the functional relevance of human tumor macrophages, we sorted human ovarian tumor–associated macrophages to conduct in vitro and in vivo immune functional experiments. We now document that a fraction of tumor macrophages that expresses B7-H4 significantly inhibits TAA-specific T cell proliferation, cytokine production, and cytotoxicity in vitro. These B7-H4+ tumor macrophages also inhibit TAA-specific immunity in vivo and foster tumor growth in chimeric SCID/NOD mice bearing autologous human tumors, despite the presence of potent TAA-specific effector T cells. The notion that tumor macrophage B7-H4 signals contribute to immunopathology is supported by several lines of evidence. First, B7-H4+ tumor macrophages are significantly more suppressive than B7-H4− tumor macrophages. Second, blocking B7-H4 on tumor-conditioned macrophages disables their suppressive capacity. Third, forced B7-H4 expression renders normal macrophages suppressive. Fourth, blocking B7-H1 and inhibiting iNOS and arginase have minor effects on B7-H4+ macrophage-mediated T cell suppression. It remains to be defined whether ovarian tumor–associated myeloid suppressor cells and ovarian tumor cells would release iNOS and arginase and, in turn, result in T cell suppression.
These findings establish B7-H4+ tumor macrophages as a novel immune regulatory population in human ovarian cancer. Our data here indicate that the suppressive potency of B7-H4+ macrophages is similar to that of CD4+ T reg cells (26). Interestingly, B7-H4+ tumor macrophages and CD4+ T reg cells, the two functionally suppressive immune cell populations, are not only physically localized in ovarian tumor environment (26), but our recent observations also suggest a mechanistic link between B7-H4+ APCs (including macrophages) and CD4+ T reg cells (27, 28). In fact, CD4+ T reg cells stimulate APC B7-H4 expression and enable APC suppressive activity through B7-H4 induction (28). B7-H4+ tumor macrophages significantly outnumber CD4+ T reg cells in ascites and the solid tumor mass. Thus, their contribution to tumor immune evasion is likely substantial. Our findings clearly establish the presence of a novel suppressor cell population in human cancer that forces a reexamination of the relative importance of T reg cells in the immunopathogenesis of cancer, and a rethinking of strategies to improve TAA-specific immunity through abrogation of suppressor cell function (2, 13–17, 50–53).
B7-H4 is a newly identified B7 family member (22–24). Although mouse B7-H4 ligation of T cells has an inhibitory effect on T cell activation (22, 23), the regulatory mechanisms and the function of human B7-H4 remain to be defined in human immunology. We show for the first time that recombinant and tumor environmental IL-6 and IL-10 stimulate monocyte/macrophage B7-H4 expression, and that GM-CSF and IL-4 reduce B7-H4 expression. We also observed similar regulatory mechanism for B7-H4 expression on myeloid dendritic cells (28). Tumor cells, tumor-associated macrophages, and T reg cells may be the source for IL-6 and IL-10 (11, 26). These data provide mechanisms for how tumor environmental IL-6 and IL-10 induce immune dysfunction. GM-CSF has been used to boost TAA-specific immunity in mouse cancer models (54, 55). A proposed mechanism for GM-CSF efficacy in these models is differentiation or attraction of DCs that boost TAA-specific immunity. In light of our present work, it will be interesting and worthwhile to reexamine these GM-CSF studies to determine whether a GM-CSF–mediated reduction in APC B7-H4 expression accounts for efficacy.
Additionally, we show for the first time that dysfunctional tumor microenvironmental cytokine balances significantly inhibit TAA-specific immunity by stimulating macrophage B7-H4 expression. This conclusion is supported by the following evidence. First, tumor macrophages, but not normal macrophages, strongly express B7-H4 that inhibits TAA-specific immunity. Second, we found high concentrations of IL-6 and IL-10, but not GM-CSF and IL-4, in the tumor microenvironment. IL-6 and IL-10 strongly stimulate macrophage B7-H4 expression, whereas IL-4 and GM-CSF strongly suppress it. Third, GM-CSF and IL-4 reduce tumor microenvironmental cytokine-induced macrophage B7-H4 expression. Fourth, tumor-conditioned macrophages impair TAA-specific T cell immunity through B7-H4 signals. Our data and those of others collectively demonstrate a new immune evasion strategy whereby tumors maximize local tolerizing conditions through suppressing DC differentiation while simultaneously inducing macrophage B7-H4 expression. These ends are mediated through maximal local accumulation of B7-H4–inducing cytokines, IL-6 and IL-10, in the virtual absence of B7-H4–reducing and DC differentiation cytokines, GM-CSF and IL-4. Strikingly, although ovarian tumor cells express B7-H4, it appears that tumor B7-H4 expression is exclusively intracellular and unable to induce T cell suppression in our in vitro culture system. Further, cytokines IL-4, GM-CSF, IL-6, and IL-10 have no regulatory effects on tumor B7-H4 expression. Although the potential in vivo pathological relevance of tumor B7-H4 remains to be defined, our data suggest that tumor B7-H4 and APC B7-H4 may be functionally distinct and differentially regulated.
In summary, our study defines the B7-H4+ macrophage as a novel suppressive cell population, and the inhibitory molecule B7-H4 is essential for their suppressive activity. The ovarian cancer microenvironment presents an overwhelming arsenal of actively tolerizing mechanisms, including an imbalance of plasmacytoid versus myeloid DCs (11), costimulatory molecules versus coinhibitory molecules (this work) (10, 25), and regulatory versus effector T cells (26). Although of important concern for the prospects to use effective immune-based therapy against ovarian cancer, these mechanisms provide many new avenues for development of novel immune-based therapies.
MATERIALS AND METHODS
Human subjects.
We studied previously untreated patients with epithelial ovarian carcinomas (International Federation of Gynecology and Obstetrics stage III or IV). Patients gave written, informed consent. The study was approved by the Tulane Institutional Review Board.
Cells and tissue biopsies.
Cells were obtained from ascites, fresh ovarian tumor tissues, and peripheral blood as described (11). CD3+ T cells and CD14+ cells were purified with paramagnetic beads (StemCell Technology) and sorted with FACSaria using DiVa software (Becton Dickinson Immunocytometry Systems). B7-H4+ and B7-H4− ascites macrophages, lin−EpCam+CD45−CD14− epithelial ovarian tumor cells were sorted on a FACSaria using DiVa software (Becton Dickinson). Cell populations were >98% pure as confirmed by flow cytometry (FACSCalibur; Becton Dickinson).
Phenotypes of tumor cells and macrophages.
The phenotypes of tumor cells and tumor ascites-conditioned macrophages were determined by RT-PCR or FACS. B7-H4 mRNA was detected by RT-PCR with specific primers: 5′-GTCGGAGCAGGATGAAATGT-3′ (sense) and 5′-CAGGAGTATGTGTTGTTGAT-3′ (antisense). The surface expression of B7-H4, CD40, CD54, CD80, CD86, B7-H1, HLA-ABC, and HLA-DR protein was detected by FACS. All the antibodies were from BD PharMingen except mouse anti–human B7-H4 (clone, hH4.1) (10).
Regulation of B7-H4 expression.
Fresh blood monocytes, tumor macrophages, and primary ovarian tumor (1–5 × 106/ml) were cultured for different time with cell-free malignant ascites, or different concentrations of recombinant human IL-10, IL-6, IL-4, and GM-CSF (R & D Systems). Neutralizing monoclonal antibody against human IL-6 (anti–IL-6, clone 6708; 500 ng/ml) and IL-10 (anti–IL-10, clone 23738; 50 ng/ml) (all from R & D Systems) and B7-H1 (clone, 5H1; 500–1000 ng/ml) (10) were used as indicated. Cells were subject to RT-PCR to detect B7-H4 mRNA and to FACS analysis to detect surface or intracellular B7-H4 protein.
Immunofluorescence analysis.
Immunofluorescence analysis was performed as described (10). Tissues were stained with polyclonal rabbit anti–human-CD3 (1/10 dilution; Dako), mouse anti–human B7-H4 (hH4.1, IgG1; 4 μg/ml) (22), mouse anti–human Ham56 (Ham56, IgM, 1/20; Dako) followed by Alexa Fluor 633–conjugated goat anti–rabbit IgG, Alexa Fluor 568–conjugated goat anti–mouse IgM, Alexa Fluor 488–conjugated goat anti–mouse IgG1, and Alexa Fluor 568–conjugated goat anti–mouse IgG2b (all 2 μg/ml; Molecular Probes). Positive cells were quantified by ImagePro Plus software and expressed as the mean of the percentage of positive cells ± SD in 10 high-powered fields using confocal microscopy.
TAA-specific T cell immunosuppression.
MDCs were differentiated from monocytes with GM-CSF and IL-4 as described (30). DCs were loaded with three distinct HLA-A2–binding Her-2/neu peptides (31) at 5 μg/ml each: p369-384 (KIFGSLAFLPESFDGDPA), p688-703 (RRLLQETELVEPLTPS), and p971-984 (ELVSEFSRMARDPQ), or HLA-A2 influenza virus matrix control peptide GILGFVFTL (Multiple Peptide System). Peptide-loaded myeloid DCs (TAA-DCs) (1–10 × 103/ml) were used to activate autologous tumor CD3+CD25− T cells (105/ml). The tumor macrophage to TAA-DC ratio was 0:1, 0.1:1, or 1:1. On day 6, TAA-specific T cell proliferation and cytokines were detected as described (10, 11). T2 cells (5 × 106/ml) (American Type Culture Collection) were labeled with 10 μm CFSE (Molecular Probes) for 10 min in the dark at 37°C. Cytotoxicity was assessed using CFSE-labeled T2 cells bearing all three Her-2/neu peptides or matrix control by annexin V staining (26, 56).
Tumor environmental cytokines.
The mRNA expressions of IL-6, IL-10, IL-4, and GM-CSF were detected by RT-PCR as described (57). IL-6, IL-10, IL-4, and GM-CSF proteins were detected by ELISA (R & D Systems).
Polyclonal T cell immunosuppression.
Polyclonal T cell immunosuppression was tested in a co-culture system. CD3+ T cells (2 × 105/ml) were stimulated with 2.5 μg/ml anti–human CD3 (BD Biosciences PharMingen) and blood monocytes (2 × 105/ml) in the presence or absence of freshly isolated tumor macrophages or conditioned macrophages as indicated. 72 h after co-culture, T cell proliferation, cellular cycle, and cytokine production were evaluated as described (10). The selective arginase inhibitor, N-hydroxy-nor-l-arginine (50 μM) (Calbiochem) and the selective iNOS inhibitor, N-monomethyl-l-arginine (1 mM) (Calbiochem) were added into some cultures to investigate macrophage-mediated suppressive mechanisms.
B7-H4 blocking experiments.
Antisense morpholino oligonucleotide specific for B7-H4 (GAGGATCTGCCCCAGGGAAGCCATG) (B7-H4 blocking oligo) and the inverted control oligonucleotide (control oligo) were produced by GeneTools. To block macrophage B7-H4, monocytes or macrophages were incubated for 3 h with 0.6 μM oligo in serum-free medium (X-VIVO 15; Biowittaker) supplemented with 0.2 μM ethoxylated polyethylenimine (GeneTools). Cells were washed twice, before use in vitro and in vivo assays.
Western blot analysis.
Macrophages were resuspended in lysis buffer (50 mM HEPES, 150 mM NaCl, 5 mM EDTA, 1 mM NaOV4, and 0.5% Triton X-100) containing 50 μg/ml aprotinin, 50 μg/ml leupeptin, 100 μg/ml trypsin-chymotrypsin inhibitor, and 2 mM PMSF. Lysates were centrifuged at 3,000 g for 10 min at 4°C. The expression of arginase and iNOS was detected by immunoblot using GAPDH as a housekeeping protein.
B7-H4 transfection.
Fresh monocytes or macrophages were transfected with 2.5 μg plasmid DNA encoding either human B7-H4 (pcDNA3.1-B7-H4), the corresponding vector plasmid (pcDNA3.1), or GFP-labeled control plasmid as directed (Nucleovector kit; Amaxa Inc.). 20 h after transfection, viable cells were subject to in vitro suppression assays.
In vivo tumor regression assay.
Primary ovarian tumor cells (107) in 200 μl of buffered saline were subcutaneously injected into dorsal tissues of female NOD.CB17-SCID mice (6–8 wk old; Jackson Laboratories) (10). Autologous tumor-specific T cells (6 × 106) (10, 26) plus macrophages (3 × 106) were injected intravenously into mice on day 12 after human tumor inoculation. Tumor size was measured twice weekly using calipers fitted with a Vernier scale. Tumor volume was calculated based on three perpendicular measurements (10, 26).
Statistical analysis.
Differences in cell surface molecule expression were determined by χ2 test and in other variables by unpaired Student's t test, with P < 0.05 being considered significant.
Online supplemental material.
Fig. S1 shows the regulation of tumor B7-H4 expression. Table S1 displays the phenotype of B7H4+ and B7H4− tumor-associated macrophages. Tumor ascites macrophages and tumor cells were isolated and sorted as described in Materials and methods. Phenotypes of fresh tumor ascites macrophages and B7-H4 in primary and cultured tumor cells were determined by FACS. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20050930/DC1.
Supplemental Material
Acknowledgments
We thank Andrew S. Flies for technical assistance.
This work was supported by the Department of Defense (OC020173), National Cancer Institute (CA092562, CA100227, CA099985), Louisiana Cancer Research Consortium (to W. Zou), and CA97085 (to L. Chen).
The authors have no conflicting financial interests.
Abbreviations used: cpm, counts per minute; iNOS, inducible nitric oxide synthase; MDC, myeloid dendritic cell; SDF, stromal-derived factor; TAA, tumor-associated antigen; T reg cell, T regulatory cell; VEGF, vascular endothelial growth factor.
L. Zou and P. Rodriguez contributed equally to this work.
References
- 1.Spiotto, M.T., P. Yu, D.A. Rowley, M.I. Nishimura, S.C. Meredith, T.F. Gajewski, Y.X. Fu, and H. Schreiber. 2002. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 17:737–747. [DOI] [PubMed] [Google Scholar]
- 2.Yu, P., Y. Lee, W. Liu, R.K. Chin, J. Wang, Y. Wang, A. Schietinger, M. Philip, H. Schreiber, and Y.X. Fu. 2004. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat. Immunol. 5:141–149. [DOI] [PubMed] [Google Scholar]
- 3.Singh, S., S.R. Ross, M. Acena, D.A. Rowley, and H. Schreiber. 1992. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J. Exp. Med. 175:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perdrizet, G.A., S.R. Ross, H.J. Stauss, S. Singh, H. Koeppen, and H. Schreiber. 1990. Animals bearing malignant grafts reject normal grafts that express through gene transfer the same antigen. J. Exp. Med. 171:1205–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou, W. 2005. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 5:263–274. [DOI] [PubMed] [Google Scholar]
- 6.Wyckoff, J., W. Wang, E.Y. Lin, Y. Wang, F. Pixley, E.R. Stanley, T. Graf, J.W. Pollard, J. Segall, and J. Condeelis. 2004. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 64:7022–7029. [DOI] [PubMed] [Google Scholar]
- 7.Pollard, J.W. 2004. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 4:71–78. [DOI] [PubMed] [Google Scholar]
- 8.Vakkila, J., and M.T. Lotze. 2004. Inflammation and necrosis promote tumour growth. Nat. Rev. Immunol. 4:641–648. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani, A., S. Sozzani, M. Locati, P. Allavena, and A. Sica. 2002. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23:549–555. [DOI] [PubMed] [Google Scholar]
- 10.Curiel, T.J., S. Wei, H. Dong, X. Alvarez, P. Cheng, P. Mottram, R. Krzysiek, K.L. Knutson, B. Daniel, M.C. Zimmermann, et al. 2003. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9:562–567. [DOI] [PubMed] [Google Scholar]
- 11.Zou, W., V. Machelon, A. Coulomb-L'Hermin, J. Borvak, F. Nome, T. Isaeva, S. Wei, R. Krzysiek, I. Durand-Gasselin, A. Gordon, et al. 2001. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat. Med. 7:1339–1346. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich, D. 2004. Mechanisms and functional significance of tumour- induced dendritic-cell defects. Nat. Rev. Immunol. 4:941–952. [DOI] [PubMed] [Google Scholar]
- 13.Pardoll, D. 2003. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 21:807–839. [DOI] [PubMed] [Google Scholar]
- 14.Cerundolo, V., I.F. Hermans, and M. Salio. 2004. Dendritic cells: a journey from laboratory to clinic. Nat. Immunol. 5:7–10. [DOI] [PubMed] [Google Scholar]
- 15.Gilboa, E. 2004. The promise of cancer vaccines. Nat. Rev. Cancer. 4:401–411. [DOI] [PubMed] [Google Scholar]
- 16.Munn, D.H., and A.L. Mellor. 2004. IDO and tolerance to tumors. Trends Mol. Med. 10:15–18. [DOI] [PubMed] [Google Scholar]
- 17.Finn, O.J. 2003. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol. 3:630–641. [DOI] [PubMed] [Google Scholar]
- 18.O'Neill, D.W., S. Adams, and N. Bhardwaj. 2004. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 104:2235–2246. [DOI] [PubMed] [Google Scholar]
- 19.Bingle, L., N.J. Brown, and C.E. Lewis. 2002. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 196:254–265. [DOI] [PubMed] [Google Scholar]
- 20.Zavadova, E., A. Loercher, S. Verstovsek, C.F. Verschraegen, M. Micksche, and R.S. Freedman. 1999. The role of macrophages in antitumor defense of patients with ovarian cancer. Hematol. Oncol. Clin. North Am. 13:135–144, ix. [DOI] [PubMed] [Google Scholar]
- 21.Ohno, S., N. Suzuki, Y. Ohno, H. Inagawa, G. Soma, and M. Inoue. 2003. Tumor-associated macrophages: foe or accomplice of tumors? Anticancer Res. 23:4395–4409. [PubMed] [Google Scholar]
- 22.Sica, G.L., I.H. Choi, G. Zhu, K. Tamada, S.D. Wang, H. Tamura, A.I. Chapoval, D.B. Flies, J. Bajorath, and L. Chen. 2003. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 18:849–861. [DOI] [PubMed] [Google Scholar]
- 23.Prasad, D.V., S. Richards, X.M. Mai, and C. Dong. 2003. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 18:863–873. [DOI] [PubMed] [Google Scholar]
- 24.Zang, X., P. Loke, J. Kim, K. Murphy, R. Waitz, and J.P. Allison. 2003. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA. 100:10388–10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, L. 2004. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 4:336–347. [DOI] [PubMed] [Google Scholar]
- 26.Curiel, T.J., G. Coukos, L. Zou, X. Alvarez, P. Cheng, P. Mottram, M. Evdemon-Hogan, J.R. Conejo-Garcia, L. Zhang, M. Burow, et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942–949. [DOI] [PubMed] [Google Scholar]
- 27.Zou, W. 2006. Regulatory T cells, tumour immunity. and immunotherapy. Nat. Rev. Immunol. 6:295–307. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek, I., S. Wei, L. Zou, G. Zhu, P. Mottram, L. Chen, and W. Zou. Induction of B7-H4 on antigen presenting cells through interleukin 10: novel suppressive mode for regulatory T cells. J. Immunol. In press. [DOI] [PubMed]
- 29.Kryczek, I., A. Lange, P. Mottram, X. Alvarez, P. Cheng, M. Hogan, L. Moons, S. Wei, L. Zou, V. Machelon, et al. 2005. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 65:465–472. [PubMed] [Google Scholar]
- 30.Zou, W., J. Borvak, F. Marches, S. Wei, P. Galanaud, D. Emilie, and T.J. Curiel. 2000. Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12. J. Immunol. 165:4388–4396. [DOI] [PubMed] [Google Scholar]
- 31.Knutson, K.L., K. Schiffman, and M.L. Disis. 2001. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J. Clin. Invest. 107:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez, P.C., A.H. Zea, J. DeSalvo, K.S. Culotta, J. Zabaleta, D.G. Quiceno, J.B. Ochoa, and A.C. Ochoa. 2003. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol. 171:1232–1239. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez, P.C., D.G. Quiceno, J. Zabaleta, B. Ortiz, A.H. Zea, M.B. Piazuelo, A. Delgado, P. Correa, J. Brayer, E.M. Sotomayor, et al. 2004. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64:5839–5849. [DOI] [PubMed] [Google Scholar]
- 34.Sinha, P., V.K. Clements, and S. Ostrand-Rosenberg. 2005. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol. 174:636–645. [DOI] [PubMed] [Google Scholar]
- 35.Kusmartsev, S., Y. Nefedova, D. Yoder, and D.I. Gabrilovich. 2004. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172:989–999. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez, P.C., C.P. Hernandez, D. Quiceno, S.M. Dubinett, J. Zabaleta, J.B. Ochoa, J. Gilbert, and A.C. Ochoa. 2005. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 202:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bronte, V., P. Serafini, E. Apolloni, and P. Zanovello. 2001. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J. Immunother. 24:431–446. [DOI] [PubMed] [Google Scholar]
- 38.Bronte, V., P. Serafini, C. De Santo, I. Marigo, V. Tosello, A. Mazzoni, D.M. Segal, C. Staib, M. Lowel, G. Sutter, et al. 2003. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170:270–278. [DOI] [PubMed] [Google Scholar]
- 39.Bronte, V., and P. Zanovello. 2005. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5:641–654. [DOI] [PubMed] [Google Scholar]
- 40.Bronte, V., T. Kasic, G. Gri, K. Gallana, G. Borsellino, I. Marigo, L. Battistini, M. Iafrate, T. Prayer-Galetti, F. Pagano, and A. Viola. 2005. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201:1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzoni, A., V. Bronte, A. Visintin, J.H. Spitzer, E. Apolloni, P. Serafini, P. Zanovello, and D.M. Segal. 2002. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168:689–695. [DOI] [PubMed] [Google Scholar]
- 42.Dong, H., S.E. Strome, D.R. Salomao, H. Tamura, F. Hirano, D.B. Flies, P.C. Roche, J. Lu, G. Zhu, K. Tamada, et al. 2002. Tumor- associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 8:793–800. [DOI] [PubMed] [Google Scholar]
- 43.Strome, S.E., H. Dong, H. Tamura, S.G. Voss, D.B. Flies, K. Tamada, D. Salomao, J. Cheville, F. Hirano, W. Lin, et al. 2003. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 63:6501–6505. [PubMed] [Google Scholar]
- 44.Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 46.Von Herrath, M.G., and L.C. Harrison. 2003. Regulatory lymphocytes: antigen-induced regulatory T cells in autoimmunity. Nat. Rev. Immunol. 3:223–232. [DOI] [PubMed] [Google Scholar]
- 47.Woo, E.Y., H. Yeh, C.S. Chu, K. Schlienger, R.G. Carroll, J.L. Riley, L.R. Kaiser, and C.H. June. 2002. Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 168:4272–4276. [DOI] [PubMed] [Google Scholar]
- 48.Mills, C.D., K. Kincaid, J.M. Alt, M.J. Heilman, and A.M. Hill. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166–6173. 10843666 [Google Scholar]
- 49.Kusmartsev, S.A., Y. Li, and S.H. Chen. 2000. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J. Immunol. 165:779–785. [DOI] [PubMed] [Google Scholar]
- 50.Hellstrom, K.E., and I. Hellstrom. 2003. Novel approaches to therapeutic cancer vaccines. Expert Rev. Vaccines. 2:517–532. [DOI] [PubMed] [Google Scholar]
- 51.Khong, H.T., and N.P. Restifo. 2002. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 3:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreiber, H., T.H. Wu, J. Nachman, and W.M. Kast. 2002. Immunodominance and tumor escape. Semin. Cancer Biol. 12:25–31. [DOI] [PubMed] [Google Scholar]
- 53.Abbud, R.A., C.K. Finegan, L.A. Guay, and E.A. Rich. 1995. Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers. J. Infect. Dis. 172:859–863. 7658083 [Google Scholar]
- 54.van Elsas, A., R.P. Sutmuller, A.A. Hurwitz, J. Ziskin, J. Villasenor, J.P. Medema, W.W. Overwijk, N.P. Restifo, C.J. Melief, R. Offringa, and J.P. Allison. 2001. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J. Exp. Med. 194:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levitsky, H.I., A. Lazenby, R.J. Hayashi, and D.M. Pardoll. 1994. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J. Exp. Med. 179:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lecoeur, H., M. Fevrier, S. Garcia, Y. Riviere, and M.L. Gougeon. 2001. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J. Immunol. Methods. 253:177–187. [DOI] [PubMed] [Google Scholar]
- 57.Zou, W., I. Durand-Gasselin, A. Dulioust, M.C. Maillot, P. Galanaud, and D. Emilie. 1995. Quantification of cytokine gene expression by competitive PCR using a colorimetric assay. Eur. Cytokine Netw. 6:257–264. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.