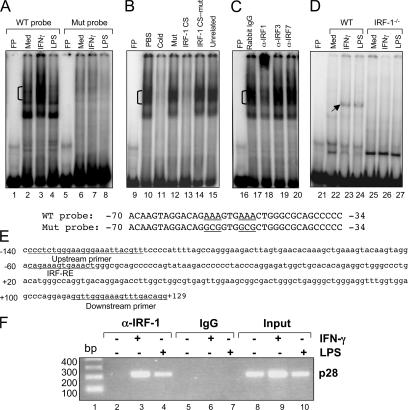
Figure 8.
IRF-1 binds to p28 promoter in vitro and in vivo. (A) Nuclear extracts were isolated from RAW264.7 cells after IFN-γ or LPS stimulation for 4 h. EMSA was performed with 10 μg of nuclear extract for each sample and a double-stranded oligonucleotide probe containing the −70/−34 region of the WT or mutant mouse p28 promoter (sequence given with the critical IRF-RE and the mutations underlined). (B) Competitive EMSA was performed with the −70/−34 probe and various competitors as indicated. (C) A supershift EMSA was performed with the −70/−34 probe and nuclear extracts from ether IFN-γ–treated RAW264.7 cells. Three IRF-related antibodies and their control rabbit IgG were used (2 μg/lane). (D) Nuclear extracts were isolated from thioglycolate-elicited primary mouse peritoneal macrophages from WT and IRF-1−/− mice after IFN-γ or LPS stimulation for 4 h. EMSA was performed with 10 μg of nuclear extract for each sample and a double-stranded oligonucleotide probe containing the −70/−34 region of the WT mouse p28 promoter. (E) Sequence of mouse p28 promoter containing IRF-RE. The sequences of the pair of PCR primers used to perform ChIP are underlined as well as the IRF-RE. (F) ChIP analysis was performed in WT mouse peritoneal macrophages. The amplified mouse genomic fragment derived from the endogenous p28 promoter encompassing the IRF-RE is indicated. The control antibody was an isotype-matched IgG. Input DNAs were used as controls.