Figure 1.
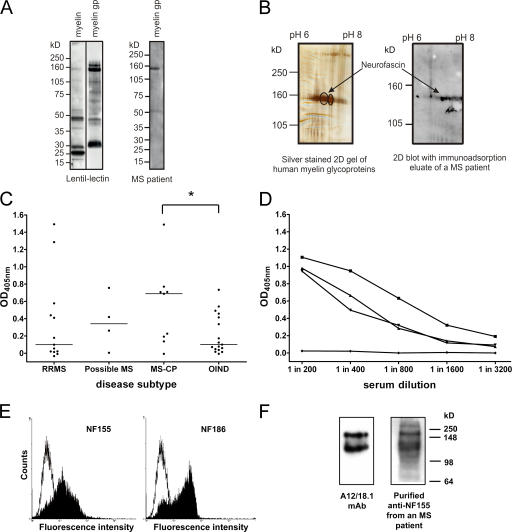
Antibodies to NF155 and NF186 are present in patients with MS. (A) Human myelin and the myelin glycoprotein (gp) fraction were separated by SDS-PAGE and blotted, and glycoproteins were detected using biotinylated lentil-lectin. The myelin glycoprotein fraction is highly enriched in higher molecular mass glycoproteins (left). Using this myelin glycoprotein fraction to screen patient sera by Western blotting identified antibody responses to a variety of components, including a protein migrating with an apparent molecular mass of ∼155 kD (right). (B) Two two-dimensional gels were run in parallel. One was silver stained (left) and the other was blotted and developed with IgG obtained from an immunoadsorption eluate of an MS patient (right). The encircled spots at 155 kD were excised and identified by MALDI-MS as neurofascin. (C) Serum IgG antibody titers to recombinant NF155ED were measured by ELISA in OIND (n = 17) and MS (n = 26) patients stratified by the disease course. Patients with chronic progressive MS (MS-CP) had the highest median antibody response (horizontal lines) to NF155ED, which was significantly higher than in OIND patients. *, P < 0.05 using the Mann-Whitney U test. (D) The titrations of three sera from high titer MS patients and one negative control donor are shown. (E) Anti-NF155ECD antibodies affinity purified from plasma of an MS patient bind to both isoforms of neurofascin when expressed at the surface of transfected cells (left, NF155; right, NF186), as shown by flow cytometry. The open graph represents the staining of the control cell line, and the shaded graph represents the staining of the neurofascin transfectants. (F) Rat brain homogenate was separated by SDS-PAGE, blotted, and probed with either the neurofascin-specific mAb A12/18.1 or the purified anti-NF155ECD antibodies, indicating recognition of both the 155- and 186-kD isoforms.