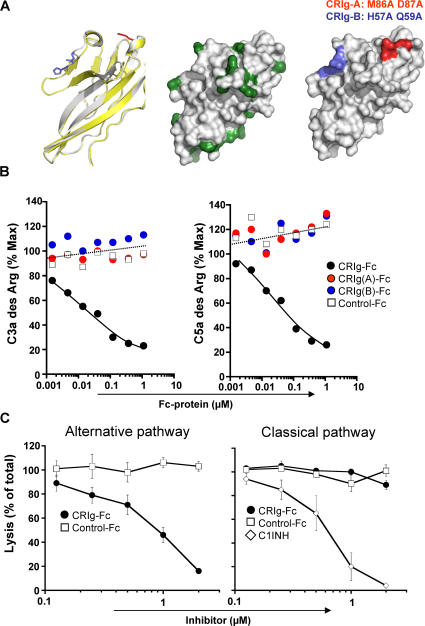
Figure 1.
Structural requirements for CRIg-Fc inhibition of the AP C3 and C5 convertases in mouse serum. (A) Left: Overlay of human (white) and murine (yellow) CRIg IgV domains. Side chains of residues M86, D87 (red) and H57, and Q59 (blue) are indicated. Middle: Surface representation of murine CRIg crystal structure. Indicated in green are surface-exposed amino acid residues that are not conserved in human and murine CRIg. Right: Surface representation of muCRIg. Indicated are amino acid residues that are substituted by alanine. Red, M86 and D87 (CRIg(A)); blue, H57 and Q59 (CRIg(B)). (B) CRIg-Fc, but not CRIg(A)-Fc, CRIg(B)-Fc, or control Fc-protein, inhibits the generation of C3a des Arg (left) and C5a des Arg (right) in zymosan-activated serum. Zymosan particles were incubated for 45 min at 37°C with 4% mouse serum in the presence of Mg2+ and EGTA. C3a des Arg and C5a des Arg were detected by ELISA and expressed as percentages of values in the absence of recombinant protein. Results are representative of four independent repeats. (C) Murine CRIg-Fc inhibits complement activation through the alternative, but not classical, pathways. Rabbit erythrocytes (left) or IgM-oponized sheep erythrocytes (right) were exposed to C1q-deficient (left) or factor B–depleted (right) human serum in the presence of increasing concentrations of CRIg-Fc or control-Fc protein. Values expressed as percent hemolysis in the absence of inhibitors (mean ± SEM; n = 3).