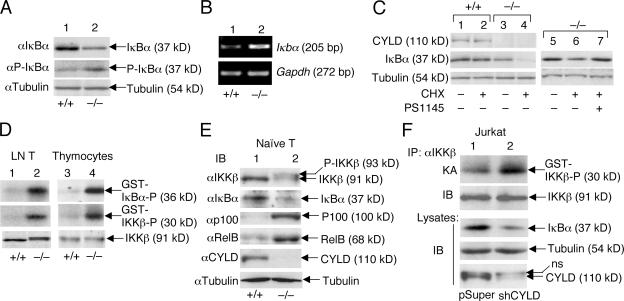
Figure 5.
Cyld deficiency results in the constitutive activation of IKKβ and chronic degradation of IκBβ. (A and B) Chronic degradation and resynthesis of IκBα in Cyld−/− T cells. Wild-type (+/+) and mutant (−/−) total T cells were subjected to IB assays (A) or RT-PCR (B) to detect the total and phosphorylated IκBα (P-IκBα) and the IκBα messenger RNA, respectively. (C) IκBα degradation in Cyld−/− T cells is mediated by IKKβ. Wild-type and mutant T cells were incubated for 50 min in the absence (–) or presence (+) of 10 μg/ml cycloheximide (CHX). In lane 7, the cells were preincubated with 10 μM of the IKKβ inhibitor PS1145 for 60 min before the start of cycloheximide treatment. The expression of IκBα as well as CYLD and tubulin was analyzed by IB. (D) Chronic activation of IKKβ in Cyld−/− T cells. IKKβ was isolated by immunoprecipitation (IP; using anti-IKKβ) from untreated wild-type (+/+) and Cyld −/− lymph node T cells and thymocytes, and the activity of IKKβ was measured by in vitro kinase assays using both GST-IκBα and GST-IKKβ substrates (top two panels). IB was performed to monitor the IKKβ protein level (bottom). (E) Whole cell extracts isolated from purified naive T cells (untreated) were subjected to IB. (F) Whole cell extracts isolated from untreated Jurkat-pSUPER and Jurkat-shCYLD cells were subjected to IKKβ kinase assay (KA; top) followed by IB to detect the IKKβ protein on the kinase assay membrane (second blot). Cell lysates were subjected to IB to detect the indicated proteins (third to fifth blots).