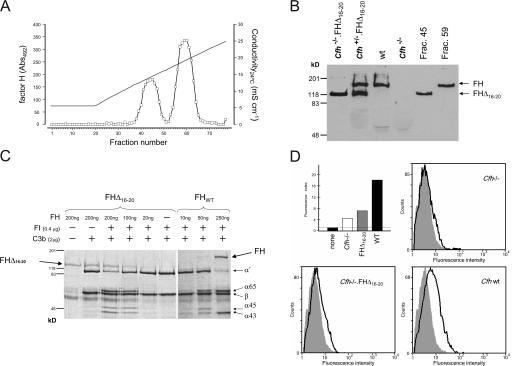
Figure 3.
Functional characterization of FHΔ16-20. (A and B) Heparin binding assay. Cfh −/+.FHΔ16-20 mouse plasma was applied to a heparin–sepharose column, and the proteins bound to the column were eluted with a NaCl linear gradient (35–250 mM). Two protein peaks containing FH identified by ELISA (A) and Western blot analysis (B) showed that the mutant FHΔ16-20 protein eluted before FH, demonstrating that removal of SCR16-20 impairs binding of FH to heparin. The continuous line in A indicates conductivity. (C) Cofactor activity of FHΔ16-20 protein in the proteolysis of fluid-phase mouse C3b by factor I. Different concentrations of either purified FH or FHΔ16-20 protein were incubated with mouse C3b in the presence of factor I. Analysis of C3b proteolytic fragments on 8% SDS-PAGE gel under reducing conditions indicated that both proteins had factor I cofactor activity with the appearance of α chain fragments (α65 and α45/43). Protein fragments were visualized using Coomassie blue staining. (D) HUVEC binding assays (background level indicated by the horizontal line; top left). HUVECs were incubated with 100 μl EDTA plasma dialyzed against 0.5× PBS (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4). Bound FH or FHΔ16-20 were detected using a rabbit anti–mouse FH antibody and a goat anti–rabbit Alexa Fluor 488–conjugated antibody. Alexa Fluor 488–conjugated isotype-matched antibody was used as a control (shaded area). The fluorescence index was calculated by multiplying the mean fluorescent intensity by the percentage of cells staining positive for FH (bold line). These analyses demonstrated that the mutant FHΔ16-20 protein has a markedly impaired ability to bind to HUVECs in comparison with wild-type protein.