Figure 5.
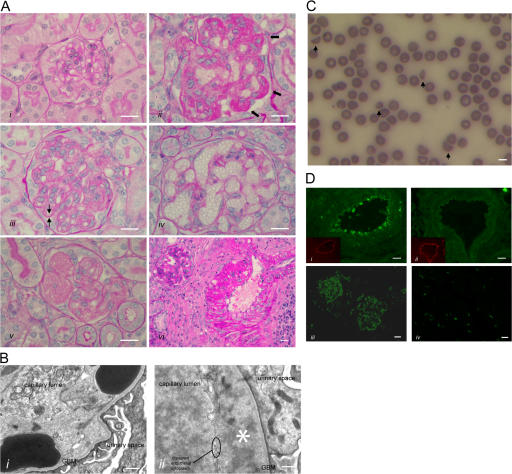
HUS in Cfh−/−.FHΔ16-20 mice. (A) Renal histology in Cfh −/−.FHΔ16-20 mice. Normal glomerulus from a 2-mo-old Cfh +/−.FHΔ16-20 mouse (i), and light microscopic features of thrombotic microangiopathy in Cfh −/−.FHΔ16-20 mice (ii–vi). These included glomerular microthrombi (ii, arrows), capillary wall double contours (iii, arrows), formation of capillary microaneurysms (iv), and mesangiolysis (v). Inflammatory changes were also seen within glomerular arteries (vi). Bar, 10 μm. (B) Electron microscopy revealed characteristic ultrastructural changes of thrombotic microangiopathy. Erythrocytes beneath disrupted endothelium and in direct contact with the GBM (i) and endothelial disruption with subendothelial accumulation of flocculent material (ii, asterisk). Note the absence of GBM electron-dense deposits, an ultrastructural feature of MPGN2 that is normally evident at this age in Cfh −/− mice (reference 12). Bar, 500 nm. (C) Peripheral blood smear from a Cfh −/−.FHΔ16-20 mouse with hematuria. Evidence of red-cell fragmentation is seen (arrows). Bar, 5 μm. (D) Renal C3 staining. C3 deposition along the endothelium and within the smooth muscle of renal arteries was present in the Cfh −/−.FHΔ16-20 (i) but not the Cfh +/−.FHΔ16-20 (ii) mice. Insets represent the staining of mouse endothelium with anti-CD31 (platelet/endothelial cell adhesion molecule). Mesangial and capillary wall C3 staining in a Cfh −/−.FHΔ16-20 mouse with HUS (iii) in contrast to an absence of abnormal glomerular C3 staining in an age-matched Cfh +/−.FHΔ16-20 mouse (iv). No abnormal renal IgG staining was present in either the Cfh −/−.FHΔ16-20 or the Cfh +/−.FHΔ16-20 mice (not depicted). Bar, 10 μm.