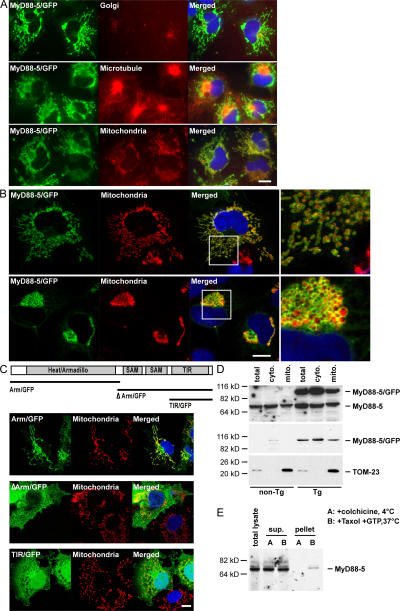
Figure 3.
Mitochondrial association of MyD88-5. (A) Selective colocalization of MyD88-5/GFP fusion protein with mitochondria and microtubules. COS-1 cells were transfected with a MyD88-5/GFP fusion construct. 18 h after the transfection, cells were stained with MitoTracker Red CMXRos for 20 min and fixed, or fixed, permeabilized, and stained with antibody for Golgi (anti–γ-adaptin) or microtubules (anti-tubulin). (B) Extrinsic association of MyD88-5 with mitochondria. Confocal microscopic images of COS-1 cells transfected with MyD88-5/GFP and stained with MitoTracker Red CMXRos. (top) Cells expressing lower levels of MyD88-5; (bottom) cells expressing higher levels of MyD88-5, with effects on mitochondrial shape and localization. (right) Magnified images of the boxed areas from the images on the left. (C) Analysis of domains of MyD88-5 contributing to subcellular localization and mitochondrial association. COS-1 cells were transfected with the indicated constructs. 18 h later, mitochondria were stained with MitoTracker Red CMXRos before fixation. (D) Partial copurification of endogenous MyD88-5 and transgenic MyD88-5/GFP with mitochondria from brain. Mitochondria were isolated from wild type (non-Tg) or transgenic (Tg) mouse brains by differential centrifugation and lysates were Western blotted with antibody to MyD88-5 (top), GFP (middle), or TOM-23 (bottom). TOM-23 is an intrinsic mitochondrial membrane protein whose relative abundance serves as a loading control. (E) Copurification of endogenous MyD88-5 and microtubules from mouse brain. Microtubules were either polymerized with Taxol and GTP at 37°C or prevented from polymerization with colchicine at 4°C before purification by sucrose gradient centrifugation. Purified microtubule fractions (pellet), unpolymerized fractions (sup), and total brain lysate (total lysate) were Western blotted with antibody to MyD88-5. Bars, 10 μm.