Abstract
Three membrane-associated proteolytic activities in Escherichia coli were resolved by DEAE-cellulose chromatography from detergent extracts of the total envelope fraction. On the basis of substrate specificity for the hydrolysis of chromogenic amino acid ester substrates, the first two eluting activities were determined previously to be protease V and protease IV, respectively (M. Pacaud, J. Bacteriol. 149:6-14, 1982). The third proteolytic activity eluting from the DEAE-cellulose column was further purified by affinity chromatography on benzamidine-Sepharose 6B. We termed this enzyme protease VI. Protease VI did not hydrolyze any of the chromogenic substrates used in the detection of protease IV and protease V. However, all three enzymes generated acid-soluble fragments from a mixture of E. coli membrane proteins which were biosynthetically labeled with radioactive amino acids. The activity of protease VI was sensitive to serine protease inhibitors. Using [3H]diisopropylfluorophosphate as an active-site labeling reagent, we determined that protease VI has an apparent molecular weight of 43,000 in polyacrylamide gels. All three membrane-associated serine proteases were insensitive to inhibition by Ecotin, and endogenous, periplasmic inhibitor of trypsin.
Full text
PDF

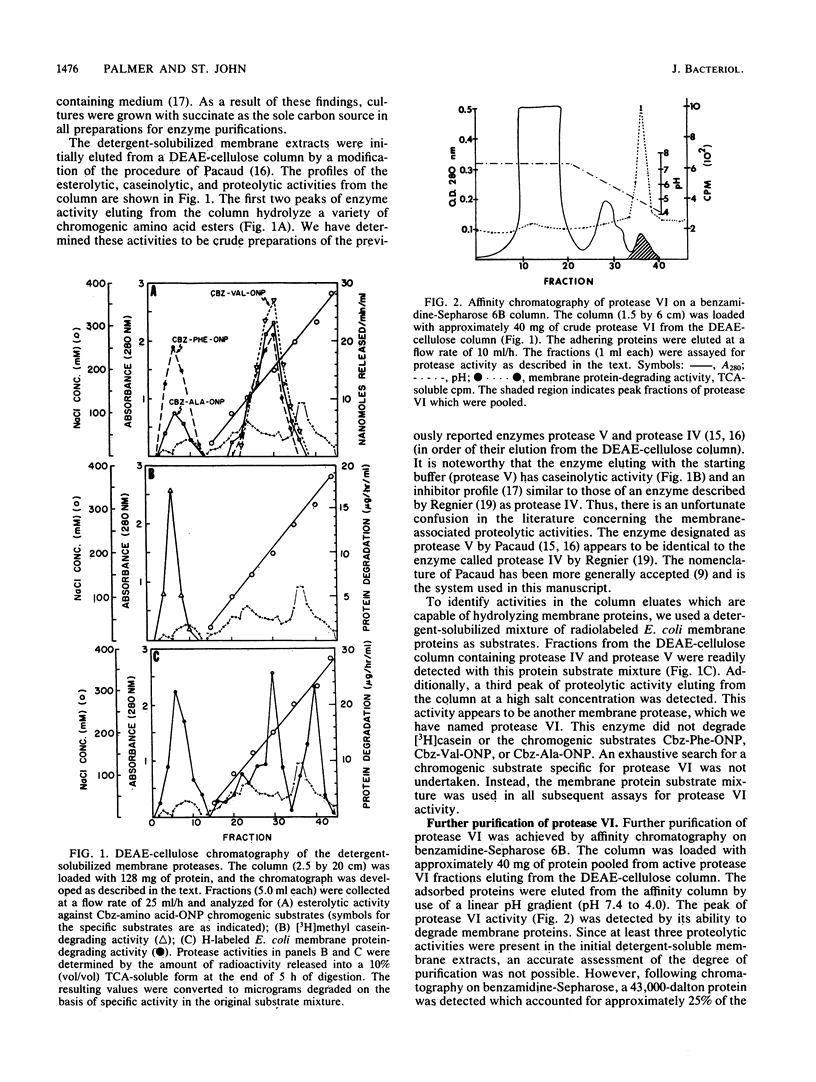
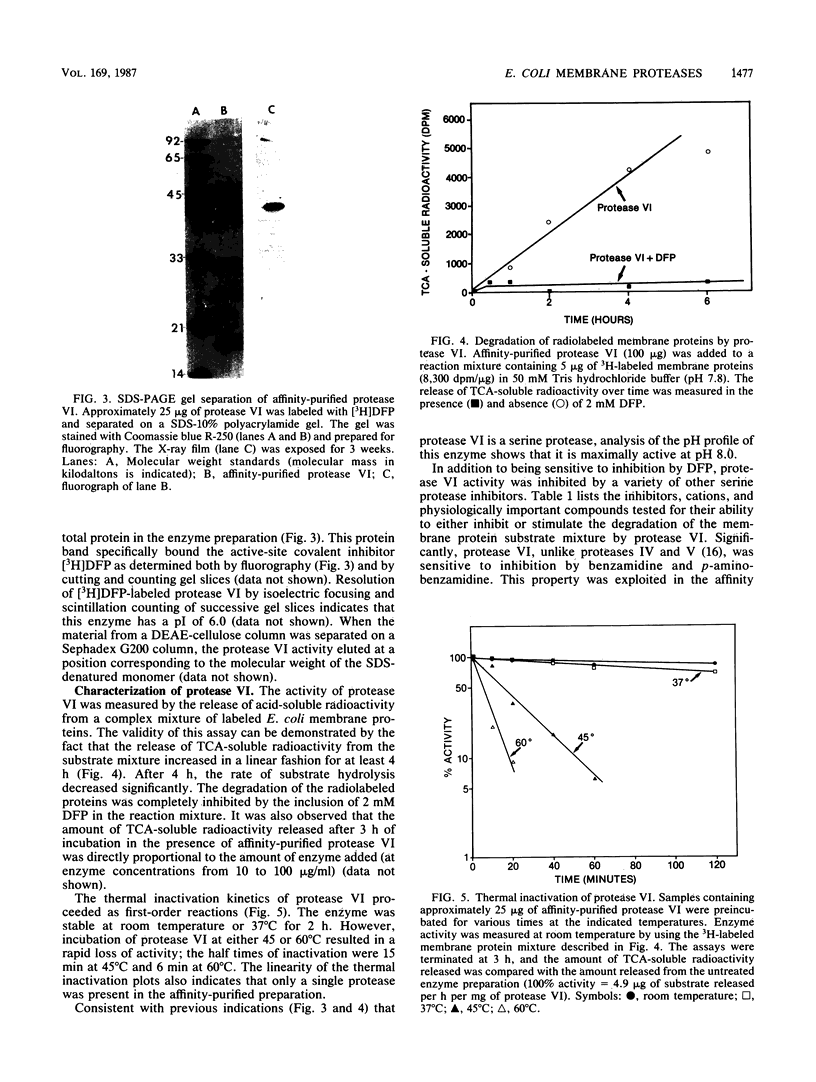


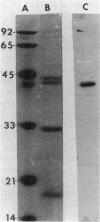
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowles L. K., Konisky J. Cleavage of colicin Ia by the Escherichia coli K-12 outer membrane is not mediated by the colicin Ia receptor. J Bacteriol. 1981 Jan;145(1):668–671. doi: 10.1128/jb.145.1.668-671.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chung C. H., Ives H. E., Almeda S., Goldberg A. L. Purification from Escherichia coli of a periplasmic protein that is a potent inhibitor of pancreatic proteases. J Biol Chem. 1983 Sep 25;258(18):11032–11038. [PubMed] [Google Scholar]
- Dev I. K., Ray P. H. Rapid assay and purification of a unique signal peptidase that processes the prolipoprotein from Escherichia coli B. J Biol Chem. 1984 Sep 10;259(17):11114–11120. [PubMed] [Google Scholar]
- Fiss E. H., Hollifield W. C., Jr, Neilands J. B. Absence of ferric enterobactin receptor modification activity in mutants of Escherichia coli K-12 lacking protein a. Biochem Biophys Res Commun. 1979 Nov 14;91(1):29–34. doi: 10.1016/0006-291x(79)90578-3. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hollifield W. C., Jr, Fiss E. H., Neilands J. B. Modification of a ferric enterobactin receptor protein from the outer membrane of Escherichia coli. Biochem Biophys Res Commun. 1978 Jul 28;83(2):739–746. doi: 10.1016/0006-291x(78)91051-3. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Beppu N., Mizushima S. Protease IV, a cytoplasmic membrane protein of Escherichia coli, has signal peptide peptidase activity. J Biol Chem. 1984 Aug 10;259(15):9853–9857. [PubMed] [Google Scholar]
- Larrabee K. L., Phillips J. O., Williams G. J., Larrabee A. R. The relative rates of protein synthesis and degradation in a growing culture of Escherichia coli. J Biol Chem. 1980 May 10;255(9):4125–4130. [PubMed] [Google Scholar]
- Leytus S. P., Bowles L. K., Konisky J., Mangel W. F. Activation of plasminogen to plasmin by a protease associated with the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1485–1489. doi: 10.1073/pnas.78.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W., Blech J. E. Localization of proteolytic activity in the outer membrane of Escherichia coli. J Bacteriol. 1979 Jan;137(1):574–583. doi: 10.1128/jb.137.1.574-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Goldstein R. V., Nishimoto K. R. Metabolism of individual proteins in exponentially growing Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2524–2532. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Identification and localization of two membrane-bound esterases from Escherichia coli. J Bacteriol. 1982 Jan;149(1):6–14. doi: 10.1128/jb.149.1.6-14.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud M. Purification and characterization of two novel proteolytic enzymes in membranes of Escherichia coli. Protease IV and protease V. J Biol Chem. 1982 Apr 25;257(8):4333–4339. [PubMed] [Google Scholar]
- Palmer S. M., St John A. C. Proteolytic activities in membranes of Escherichia coli. Prog Clin Biol Res. 1985;180:295–297. [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Rupprecht K. R., Gordon G., Lundrigan M., Gayda R. C., Markovitz A., Earhart C. omp T: Escherichia coli K-12 structural gene for protein a (3b). J Bacteriol. 1983 Feb;153(2):1104–1106. doi: 10.1128/jb.153.2.1104-1106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier P. The purification of protease IV of E. coli and the demonstration that it is an endoproteolytic enzyme. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1369–1376. doi: 10.1016/0006-291x(81)90770-1. [DOI] [PubMed] [Google Scholar]
- Schroer D. W., St John A. C. Relative stability of membrane proteins in Escherichia coli. J Bacteriol. 1981 May;146(2):476–483. doi: 10.1128/jb.146.2.476-483.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Jakubas K., Beim D. Degradation of proteins in steady-state cultures of Escherichia coli. Biochim Biophys Acta. 1979 Sep 3;586(3):537–544. doi: 10.1016/0304-4165(79)90044-8. [DOI] [PubMed] [Google Scholar]
- St John A. C., Schroer D. W., Cannavacciuolo L. Relative stability of intracellular proteins in bacterial cells. Acta Biol Med Ger. 1981;40(10-11):1375–1384. [PubMed] [Google Scholar]
- Tokunaga M., Loranger J. M., Wu H. C. Isolation and characterization of an Escherichia coli clone overproducing prolipoprotein signal peptidase. J Biol Chem. 1983 Oct 25;258(20):12102–12105. [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Purification and characterization of leader (signal) peptidase from Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7973–7977. [PubMed] [Google Scholar]