Abstract
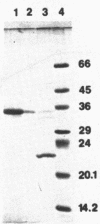
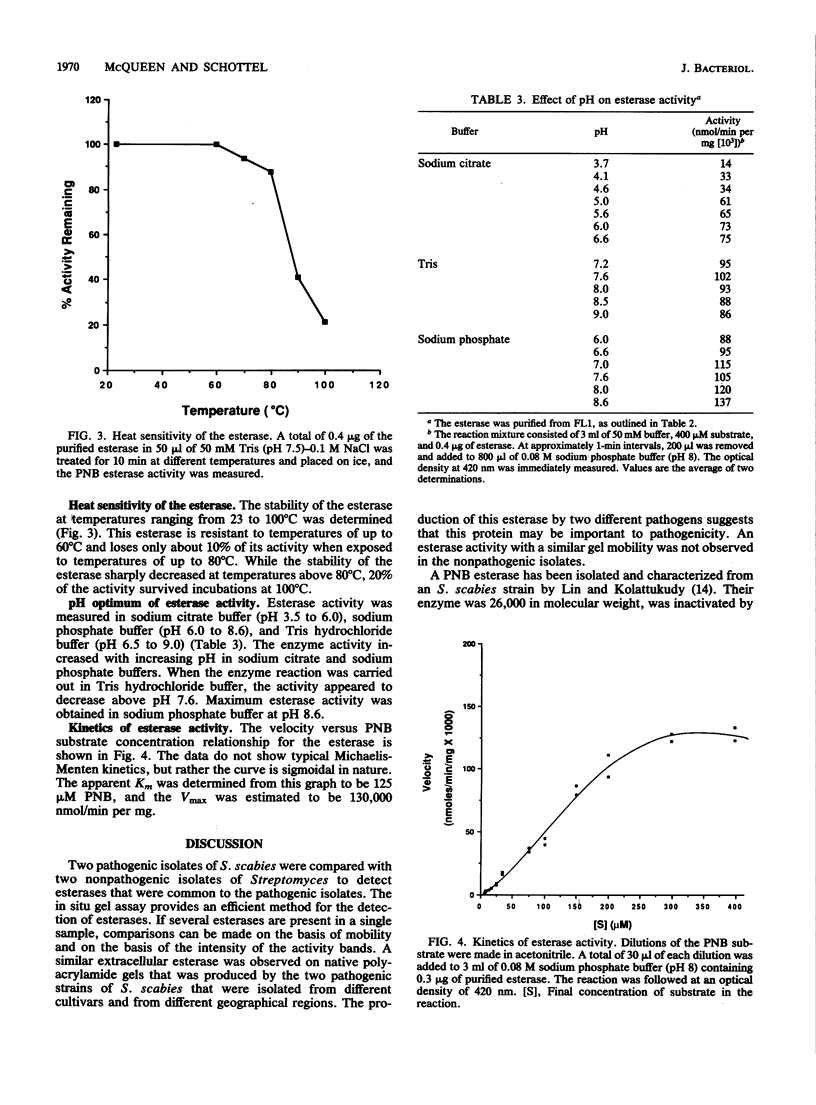
Native polyacrylamide gels of extracellular proteins produced by several Streptomyces isolates grown with suberin were assayed in situ for esterase activity. Two pathogenic isolates of Streptomyces scabies from different geographical regions were found to produce a similar esterase activity that was not produced by nonpathogenic strains. After treatment with EDTA, suberin no longer induced esterase production. Expression was restored when EDTA-treated suberin was supplemented with zinc. The optimal concentration of zinc required for esterase production was 2 microM. This esterase was purified from one of the pathogenic isolates and characterized. The enzyme was 38,000 daltons when determined by gel filtration on Sephadex G-100 and 36,000 daltons when determined by denaturing polyacrylamide gel electrophoresis. The esterase showed maximal activity in sodium phosphate buffer above pH 8.0, was stable to temperatures of up to 60 degrees C, and had an apparent Km of 125 microM p-nitrophenyl butyrate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maiti I. B., Kolattukudy P. E. Prevention of fungal infection of plants by specific inhibition of cutinase. Science. 1979 Aug 3;205(4405):507–508. doi: 10.1126/science.205.4405.507. [DOI] [PubMed] [Google Scholar]
- Okanishi M., Suzuki K., Umezawa H. Formation and reversion of Streptomycete protoplasts: cultural condition and morphological study. J Gen Microbiol. 1974 Feb;80(2):389–400. doi: 10.1099/00221287-80-2-389. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Depolymerization of a hydroxy fatty acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani f. pisi: isolation and some properties of the enzyme. Arch Biochem Biophys. 1973 Nov;159(1):61–69. doi: 10.1016/0003-9861(73)90429-3. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Roegner V., Becker F. F. The quantitation of rat serum esterases by densitometry of acrylamide gels stained for enzyme activity. Anal Biochem. 1975 May 26;66(1):206–212. doi: 10.1016/0003-2697(75)90738-1. [DOI] [PubMed] [Google Scholar]
- TROWBRIDGE C. G., KREHBIEL A., LASKOWSKI M., Jr SUBSTRATE ACTIVATION OF TRYPSIN. Biochemistry. 1963 Jul-Aug;2:843–850. doi: 10.1021/bi00904a037. [DOI] [PubMed] [Google Scholar]
- Walton T. J., Kolattukudy P. E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas chromatography and mass spectrometry. Biochemistry. 1972 May 9;11(10):1885–1896. doi: 10.1021/bi00760a025. [DOI] [PubMed] [Google Scholar]