Abstract
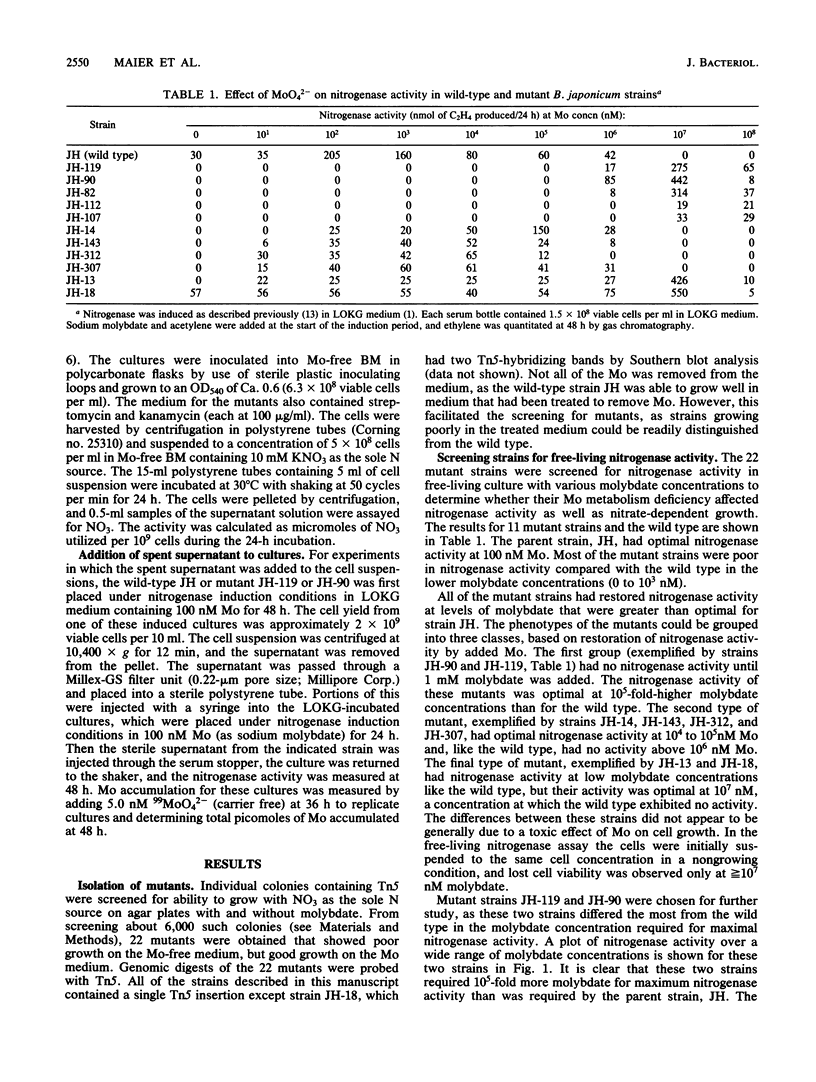
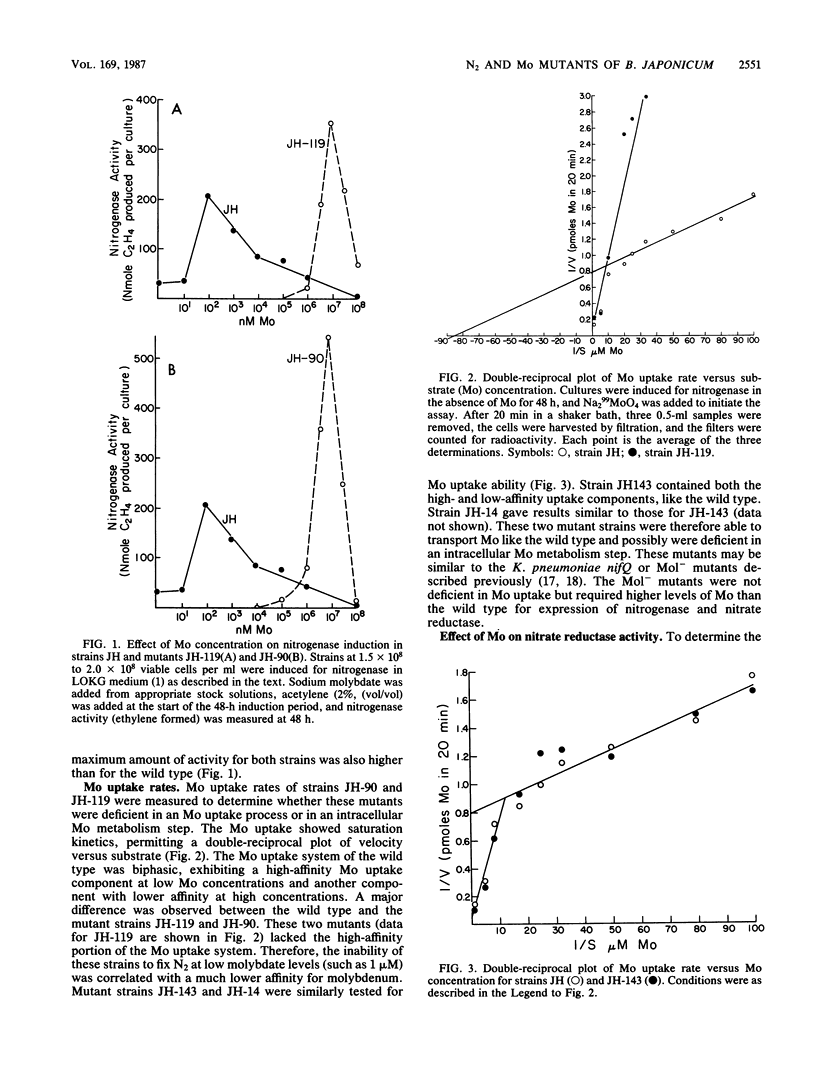
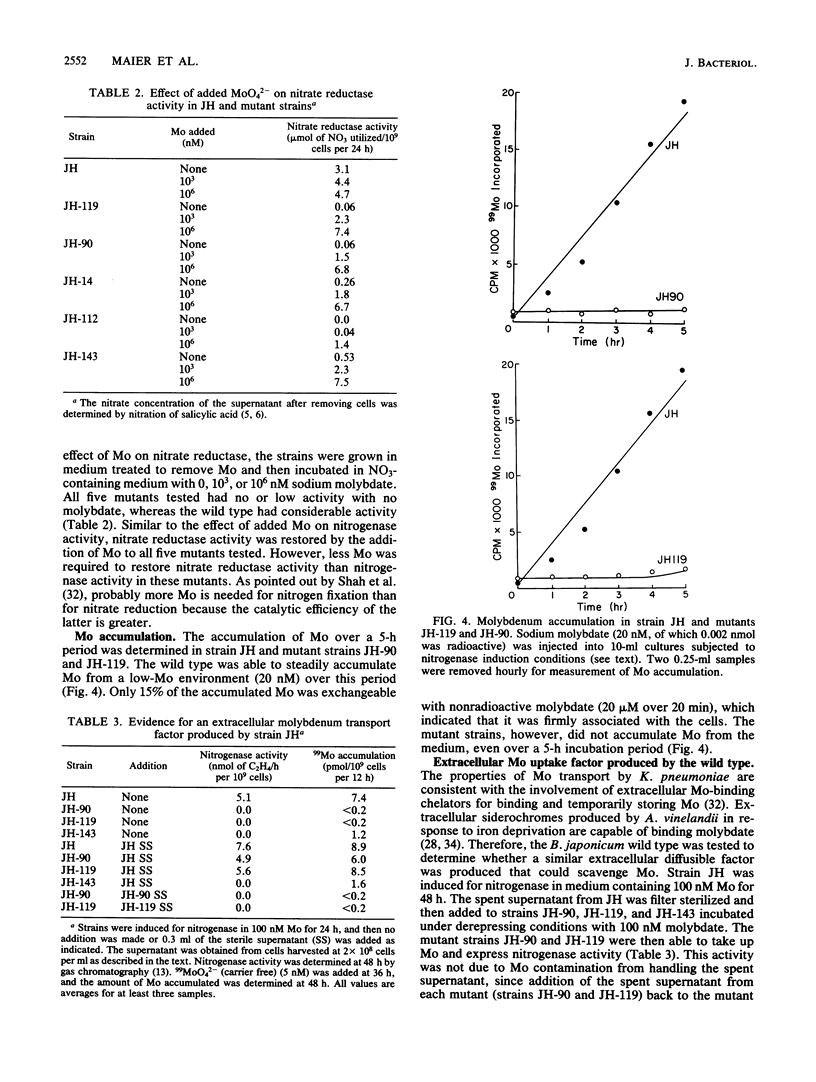
Bradyrhizobium japonicum JH mutants deficient in molybdenum metabolism into the enzymes nitrogenase and nitrate reductase were isolated by using the vector pSUP1011, which carries transposon Tn5 (streptomycin and kanamycin resistance). Mutants in Mo metabolism were obtained at a frequency of 3.6 X 10(-3) (per Kan Strr colony). The mutants were detected by their poor ability to grow in nitrate-containing medium without added Mo. One of the mutant types required 10(5) times more molybdate than the wild type to obtain maximal nitrogen fixation activity. Double-reciprocal plots of Mo uptake versus concentration indicated that the wild-type strain had a high- and a lower-affinity component for Mo binding. Mutant strains JH-90 and JH-119 lacked the high-affinity Mo uptake component and were also clearly deficient in Mo accumulation into a nonexchangeable form. Nitrogenase activity as well as Mo uptake ability could be restored in strains JH-90 and JH-119 by the addition of the sterile supernatant fraction of the wild type. Therefore, mutant strains JH-90 and JH-119 appeared to be deficient in an extracellular Mo-binding factor produced by the wild type. Mutant strains JH-14 and JH-143 had Mo uptake kinetics like those of the wild type (both high- and low-affinity binding for Mo) and appeared to be deficient in intracellular Mo metabolism processes. The addition of the wild-type supernatant did not restore Mo uptake or nitrogenase activity in these strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal A. K., Keister D. L. Physiology of Ex Planta Nitrogenase Activity in Rhizobium japonicum. Appl Environ Microbiol. 1983 May;45(5):1592–1601. doi: 10.1128/aem.45.5.1592-1601.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B. B., Mortenson L. E. Regulation of molybdate transport by Clostridium pasteurianum. J Bacteriol. 1976 Aug;127(2):770–779. doi: 10.1128/jb.127.2.770-779.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew D. L., Welch R. M., Cary E. E. A simple plant nutrient solution purification method for effective removal of trace metals using controlled pore glass-8-hydroxyquinoline chelation column chromatography. Plant Physiol. 1984 Sep;76(1):103–105. doi: 10.1104/pp.76.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton S. M., Mortenson L. E. Identification of molybdoproteins in Clostridium pasteurianum. J Bacteriol. 1985 May;162(2):477–484. doi: 10.1128/jb.162.2.477-484.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton S. M., Mortenson L. E. Regulation and order of involvement of molybdoproteins during synthesis of molybdoenzymes in Clostridium pasteurianum. J Bacteriol. 1985 May;162(2):485–493. doi: 10.1128/jb.162.2.485-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Mol- mutants of Klebsiella pneumoniae requiring high levels of molybdate for nitrogenase activity. J Bacteriol. 1985 Sep;163(3):1285–1287. doi: 10.1128/jb.163.3.1285-1287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Role of the nifQ gene product in the incorporation of molybdenum into nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1984 Apr;158(1):187–194. doi: 10.1128/jb.158.1.187-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Premakumar R., Bishop P. E. Transcriptional regulation of nitrogen fixation by molybdenum in Azotobacter vinelandii. J Bacteriol. 1986 Aug;167(2):480–486. doi: 10.1128/jb.167.2.480-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Postgate J. R. Expression of Klebsiella pneumoniae nitrogen fixation genes in nitrate reductase mutants of Escherichia coli. J Gen Microbiol. 1977 Feb;98(2):551–557. doi: 10.1099/00221287-98-2-551. [DOI] [PubMed] [Google Scholar]
- Ketchum P. A., Owens M. S. Production of molybdenum-coordinating compound by Bacillus thuringiensis. J Bacteriol. 1975 May;122(2):412–417. doi: 10.1128/jb.122.2.412-417.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Iron- and molybdenum-repressible outer membrane proteins in competent Azotobacter vinelandii. J Bacteriol. 1982 Jul;151(1):237–242. doi: 10.1128/jb.151.1.237-242.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Brill W. J. Molybdenum accumulation and storage in Klebsiella pneumoniae and Azotobacter vinelandii. J Bacteriol. 1981 Feb;145(2):743–751. doi: 10.1128/jb.145.2.743-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Burgess B. K., Dean D. R. Activity, reconstitution, and accumulation of nitrogenase components in Azotobacter vinelandii mutant strains containing defined deletions within the nitrogenase structural gene cluster. J Bacteriol. 1986 Apr;166(1):180–186. doi: 10.1128/jb.166.1.180-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Ugalde R. A., Imperial J., Brill W. J. Molybdenum in nitrogenase. Annu Rev Biochem. 1984;53:231–257. doi: 10.1146/annurev.bi.53.070184.001311. [DOI] [PubMed] [Google Scholar]


