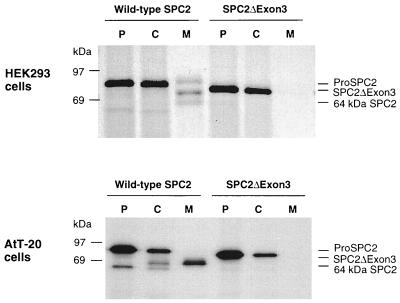
Figure 3.
Biosynthesis of normal and mutant SPC2 in cultured cells. HEK293 cells or AtT-20 cells were stably transfected with constructs encoding wild-type SPC2 or mutant SPC2 (SPC2ΔExon3) (39). Cells were labeled with [35S]methionine [1 mCi/ml; 1,000 Ci/mmol; 1 Ci = 37 GBq] for 30 min and then chase-incubated in nonradioactive medium for 3 hr at 37°C. At the termination of the incubation, media were collected and cells were lysed with a N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid/Mannitol buffer (40). Cell extracts and media samples were immunoprecipitated with SPC2 C-terminal antiserum and then analyzed by SDS/PAGE followed by fluorography. The mutant protein was not cleaved or secreted, in contrast to wild-type SPC2. To investigate whether the lack of processing activity of SPC2ΔExon3 in HEK293 cells was due to the lack of 7B2, a neuroendocrinal-specific protein that is involved in the transport and activation of SPC2 (41, 42), wild-type SPC2 and SPC2ΔExon3 were also stably transfected into AtT-20 cells, a corticotrope cell line that endogenously expresses 7B2. Pulse–chase metabolic labeling yielded similar results in this endocrine cell line. SPC2ΔExon3 appeared to be unstable and to undergo more rapid intracellular degradation without detectable secretion. P, pulse cell lysates; C, chase cell lysates; M, chase media.