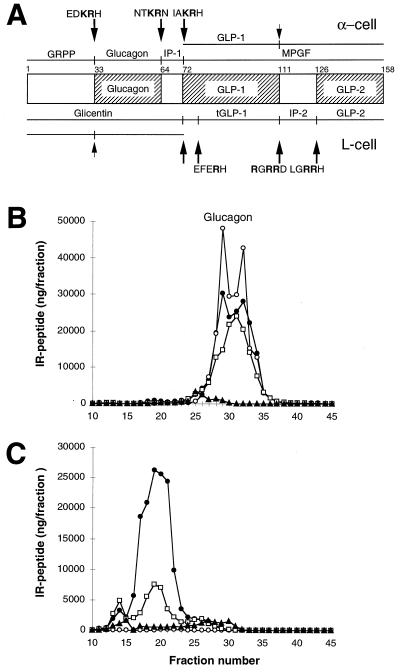
Figure 5.
Differential processing of proglucagon in the normal pancreas (α-cell) and the intestine (L-cell), and results of radioimmunoassay of glucagon in pancreas extracts. (A) Schematic representation of the structure and processing of proglucagon (20, 24, 25). At the top are the peptides resulting from proglucagon processing at the sites indicated in the pancreatic alpha cell. At the bottom are the peptides resulting from processing at the indicated sites in the intestinal L cell. These peptides include the glicentin-related polypeptide (GRPP, [proglucagon-(1–30)]), glucagon [proglucagon-(33–61)], the intervening peptide 1 (IP-1, [proglucagon-(64–69)]), the major proglucagon fragment (MPGF, [proglucagon-(72–158)]), GLP-1 [proglucagon-(72–107)], glicentin [proglucagon-(1–69)], truncated GLP-1 (tGLP-1, [proglucagon-(78–107)]), intervening peptide 2 (IP-2, [proglucagon-(111–122)]), GLP-2 [proglucagon-(126–158)], and oxyntomodulin [proglucagon-(33–69)]. The cleavage sites are indicated by arrows, with the sequence surrounding the site shown above or below the arrow. (Note that all cleavages are to the right of residues shown in bold.) Partially processed sites are indicated by small arrows. B and C show gel filtration profiles of extracts of wild-type (B) and SPC2−/− (C) pancreas analyzed using antibodies against four different regions of proglucagon. Antiserum 4304 (•) recognizes the 6–15 sequence of glucagon; 4305 (○) recognizes only the free, unextended form of glucagon; 4830 (□) was raised against the N terminus of glucagon, but shows some crossreaction with N-terminally extended forms of glucagon; 2135 (▴) recognizes a C-terminal immunodeterminant of GLP-1 and also reacts with MPGF (43).