Abstract
Conventional resuscitation (CR) from hemorrhagic shock causes a persistent and progressive splanchnic vasoconstriction and hypoperfusion despite hemodynamic restoration with intravenous fluid therapy. Adjunctive direct peritoneal resuscitation (DPR) with a clinical peritoneal dialysis solution instilled into the peritoneal cavity has been shown to restore splanchnic tissue perfusion, down-regulate the gut-derived exaggerated systemic inflammatory response, promote early fluid mobilization, and improve overall outcome. This study was conducted to define the molecular mechanisms of DPR-induced gut hyperperfusion after hemorrhagic shock. Male rats were bled to 50% baseline mean arterial pressure and resuscitated with the shed blood plus two volumes of saline (CR). In vivo videomicroscopy and Doppler velocimetry were used to assess terminal ileal microvascular diameters and blood flow. Direct peritoneal resuscitation animals received CR and topical application of a clinical glucose-based peritoneal dialysis solution (Delflex). Inhibitors, glibenclamide (K+ATP channels), N-monomethyl-l-arginine (l-NMMA) (nitric oxide synthase), 8-cyclopentyl-1,3-diprophylxanthine (DPCPX) (A1 adenosine receptor), tetrabutylammonium (K+Ca2+ channels), and mefenamic acid (cyclooxygenase) were topically applied (individually or in combination) with DPR according to protocol; BQ-123 (endothelin A receptor antagonist) and BQ-788 (endothelin B receptor antagonist) were used topically with CR to define the mechanism of post-CR vasoconstriction and hypoperfusion. Conventional resuscitation caused a persistent progressive intestinal vasoconstriction and hypoperfusion that can be abolished with endothelin antagonists. In contrast, adjunctive DPR caused an instantaneous sustained vasodilation and hyperperfusion. Glibenclamide or l-NMMA partially attenuated DPR-induced vasodilation, whereas the addition of DPCPX to the two inhibitors eliminated the dilation. Cyclooxygenase and K+Ca2+channels were not active in DPR-mediated microvascular effects. In conclusion, DPR improves splanchnic tissue perfusion by endothelium-dependent mechanisms mediated by activations of glibenclamide-sensitive K+ channels (KATP), adenosine A1 receptor subtype activation, and nitric oxide release. Direct peritoneal resuscitation preserves endothelial dilatory functions, thereby overriding any endothelium-derived constrictor response triggered by hemorrhagic shock and CR.
Keywords: Hemorrhagic shock, direct peritoneal resuscitation, vascular endothelium, vasodilation, nitric oxide, endothelin
Introduction
High morbidity and mortality from hemorrhagic shock and subsequent multiple organ failure remain a very significant and costly clinical problem (1). Restoration of central hemodynamics with aggressive fluid therapy as an end point in resuscitation is associated with a persistent and progressive splanchnic vasoconstriction and hypoperfusion (2, 3). Hemorrhagic shock causes physiological compensatory mechanisms aimed at maintaining adequate blood pressure. These mechanisms are mediated by neurohormonal reflexes through hypotension-mediated baroreceptor firing decrease, which increases sympathetic-induced vasopressor hormones such as the renin-angiotensin-aldosterone systems, vasopressin, and epinephrine, which promotes vasoconstriction and increases water retention to increase blood pressure and cardiac output. In addition, the concomitant cerebral ischemia associated with hypovolemia is known to produce a sympathetic discharge that is severalfold greater than the maximal sympathetic activation caused by the baroreceptors reflex. Other compensatory mechanisms to shock include a paradoxical fluid shift into the cellular compartment including vascular endothelial cells (4). Clinically, fluid resuscitation and intravascular volume restoration complement the physiological compensatory mechanisms to restore central hemodynamics as demonstrated by the restoration and maintenance of mean arterial pressure (MAP), cardiac output, and urine output with fluid therapy.
Two separate clinical studies document that despite normalization of blood pressure, heart rate (HR), and urine output, tissue hypoperfusion persists in 80% to 85% of patients, as evidenced by lactic acidemia and decreased mixed venous oxygen saturation (5, 6). Other clinical studies have shown that the level and rate of normalization of serum lactate (index of tissue oxygen utilization) correlated with mortality both in degree of elevation and in the time-dependent rate of normalization (7, 8). Systemic base deficit (index of tissue perfusion) also shows a similar predictive pattern of mortality (9). However, interventions that focus on correction of this oxygen debt by driving oxygen transport variables, such as cardiac index or oxygen delivery index after conventional resuscitation (CR), to supernormal levels fail to reduce mortality in severely injured patients (10, 11). Similarly, in animal models of shock/resuscitation in which splanchnic vasculature and blood flow were directly observed, progressive vasoconstriction and hypoperfusion were observed even with adequate CR that restores and maintains central hemodynamics (3, 12, 13). These studies suggest that adequate resuscitation and end points of resuscitation should be redefined according to the ability of the resuscitation regimens to restore adequate end-organ perfusion.
Recently, we introduced a new resuscitation technique to specifically target the progressive splanchnic vasoconstriction and hypoperfusion after CR from hemorrhagic shock (14, 15). Direct peritoneal resuscitation (DPR) requires the instillation of a clinical peritoneal dialysis solution into the peritoneal cavity as an adjunct to conventional fluid resuscitation. These clinical solutions are vasoactive because of their hyperosmolality and glucose and lactate contents (16, 17). Direct peritoneal resuscitation reverses the splanchnic hypoperfusion noted after CR to a state of instantaneous and sustained hyperperfusion regardless of the timing of therapy initiation (18). This improved end-organ perfusion was associated with down-regulation of the gut-derived systemic inflammatory response, promotion of early fluid mobilization, and prevention of edema formation to collectively result in a 100% 72-h survival compared with a 40% mortality with CR alone (19). The purpose of the present study is to investigate the molecular mechanisms of DPR-mediated hyperperfusion.
Methods
Animal preparation
Male Sprague-Dawley rats (195 – 215g) were used in the experiments. Animals were housed in an AAALAC-approved facility with controlled temperature and humidity according to the international Association for Assessment and Accreditation of Laboratory animal care. The experiments were performed in adherence to the National Institutes of Health Guidelines on the use laboratory Animals and the study was approved by our Institutional Animal Care and Use Committee. Animals were fasted overnight to minimize the presence of digestion products in the intestine during the experiments, but water was allowed ad libitum. Anesthesia was induced with intraperitoneal pentobarbital (60 mg/kg), and a subcutaneous supplemental dose (20% of the initial dose) was given hourly to maintain a surgical plane of anesthesia. Two milliliters of normal saline was given subcutaneously before the surgical preparation for fluid compensation. Tracheotomy was performed to reduce airway resistance, and the animal was allowed to breathe spontaneously. The right carotid artery was cannulated to monitor MAP and HR throughout the experiment (Digi-Med Signal Analyzers, Louisville, Ky). The left femoral artery and vein were cannulated for blood withdrawal and for resuscitation, respectively. Body temperature was maintained at 37.0°C ± 0.5°C with a rectal probe and feedback-controlled heating pad.
Intestinal microvascular preparation
A median abdominal laparotomy (1.5 cm) was performed. Using the cecum as a landmark, a segment (2 cm) of the terminal ileum was exteriorized with its neurovascular supply intact. Both ends of the ileal segment were ligated to exclude collateral circulation. The segment was then opened along the antimesenteric border with electrocautery, and the intestinal contents were gently removed from the lumen. The animals were placed on a specially designed polyurethane board, and the ileal segment, serosal side up, was positioned over an optical port for transillumination. Tissue bath temperature was maintained at 37.0°C ± 0.5°C throughout the experiment with feedback-controlled heating coils, and pH was maintained at 7.40 ± 0.05 with continuous bubbling of the tissue bath with CO2 and N2. The animal was placed on a stage of an upright trinocular Zeiss microscope for direct in vivo microscopy. The microvascular images were transmitted to a digital camera (Models K-P-D51/D50, Hitachi Denshi, Tokyo, Japan) and displayed in a high-resolution computer monitor. The digitized images were recorded with an AT and T image program for off-line measurements of vascular diameters with a caliper. A Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, College Station, Tex) was used to measure centerline red blood cell velocity for the calculation of the microvascular blood flow of the feed inflow arteriole from the equation: flow (nL/s) = (V/1.6)(πr2)(0.001), in which V is the centerline red blood cell velocity (in millimeters per second) and r is the vessel radius (in micrometers).
Intestinal microvascular anatomy
The microvascular anatomy was identified according to the nomenclature of Bohlen and Gore (20). Briefly, first-order arterioles (A1) branch from mesenteric arcade artery acting as feeding arterioles to the whole intestinal wall; second-order arterioles (A2) branch from A1 arterioles acting as transitional arterioles within the submucosal layer; and third-order arterioles branch at right angles from A2 arterioles acting as premucosal arterioles to supply intestinal villi. First-order venules (V1) are parallel to A1 arterioles.
Chemicals and solutions
Solution A was a modified nonglucose nonvasoactive Krebs solution, containing 6.92 g/L sodium chloride, 0.44 g/L potassium chloride, 0.37 g/L calcium chloride, and 2.1 g/L sodium bicarbonate at a pH of 7.4 and osmolarity of 285 mOsm/L. Solution B was a clinical peritoneal dialysis solution (2.5% Delflex, Fresenius USA, Inc., Ogden, Utah) that contained 5.67 g/L sodium chloride, 3.92 g/L sodium lactate, 0.257 g/L calcium chloride, and 0.152 g/L magnesium chloride at pH 5 to 6 and an osmolality of 398 mOsm/L. All chemicals were purchased from the Sigma Chemical Company (St. Louis, Mo) unless mentioned otherwise. The chemicals were ATP-dependent potassium (K+ATP) channel inhibitor, glibenclamide, 20 μmol/L; adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 200 nmol/L; nitric oxide (NO) synthase inhibitor, N-monomethyl-l-arginine (l-NMMA),100 μmol/L; calcium-dependent potassium (K+Ca2+) channel inhibitor, tetrabutylammonium, 3 mmol/L; cyclooxygenase inhibitor, mefenamic acid, 40 μmol/L; and endothelin receptor A inhibitor, BQ-123, endothelin receptor B inhibitor BQ-788. The concentration of each of the blockers, which is specific and effective against the designated pathway, has been obtained from published data and used in our previous studies. The DPCPX concentration used in our previous studies represents at least three times the 50% effective inhibitory concentration (IC50) for adenosine A1 receptor as determined by information provided by the manufacturer.
Hemorrhagic shock model
A fixed-pressure hemorrhage shock model was used in this study. Animals were bled from the femoral artery with blood withdrawal rate at 1 mL/min until the MAP dropped and maintained at 50% of baseline. The syringe used for blood withdrawal was prerinsed with 6 U heparin (1,000 U/mL) to prevent blood clotting. The nominal 50% MAP was maintained for 60 min with further blood withdrawal or reinfusion as required. Conventional resuscitation was initiated with the return of the shed blood via the femoral vein over 5 min, followed by saline (twice the shed blood volume) infusion during the next 25 min.
Experimental protocol
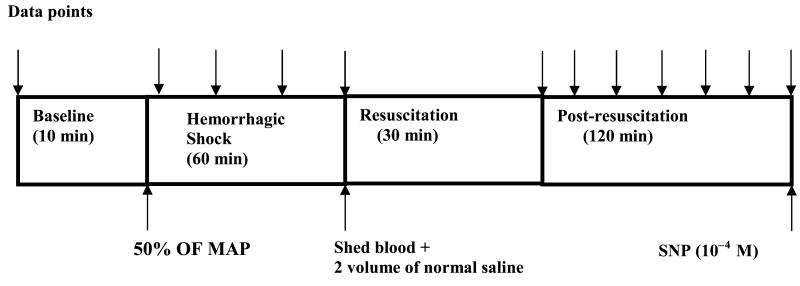
The timeline of the experimental protocol is shown in Figure 1. Forty-five minutes was allowed for animals to equilibrate after surgery during which time the ileum was continuously suffused with solution A. Baseline hemodynamics and microvascular measurements were considered valid when the variability between two successive measurements at 10-min interval was less than 0.5%. After baseline measurements were obtained, hemorrhage to 50% MAP was induced and maintained for 60 min. After the 60-min hypovolemia, CR was initiated according to protocol. To simulate DPR, solution A was withdrawn from the tissue bath, and solution B (Delflex) was instilled into the tissue bath with a 60-mL syringe. Inhibitors were administered in the tissue bath 10 min before resuscitation and remained during the rest period of the experiment. At the end of the experiment, a single dose of sodium nitroprusside (10−4 mol/L) was topically applied in the tissue bath to assess the maximal dilation capacity of the intestinal microvasculature.
Fig. 1. Experimental protocol.
Each data point measurements (arrow) consisted of MAP, HR, A1, A2, proximal premucosal third-order arteriole, distal premucosal third-order arteriole and V1 diameters, and centerline red cell velocity in the A1 arterioles. MAP indicates mean arterial pressure; SNP, sodium nitroprusside.
Experimental groups
Animals were randomly assigned to each of the following eight experimental groups (eight in each group):
Group I = DPR,
Group II = CR,
Group III = DPR + glibenclamide,;
Group IV = DPR + l-NMMA,
Group V = DPR + glibenclamide + l-NMMA + DPCPX,
Group VI = DPR + mefenamic acid,
Group VII = DPR + tetrabutylammonium, and
Group VIII = CR + BQ123 + BQ788.
Statistical analysis
All data are presented as mean ± SE. Vascular diameter and blood flow changes were presented as percentage change from baseline. Two-way analysis of variance (ANOVA) and Bonferroni posttest were used to assess differences among groups. A result was considered statistically significant at P < 0.05.
Results
There were no significant differences among experimental groups in baseline hemodynamic parameters, microvascular blood flow, or diameter data (Table 1). This warrants the validity for group comparison. As expected, MAP decreased to 50% of the baseline prehemorrhage value and was fully restored and maintained to baseline during the postresuscitation period without additional fluid therapy in all experimental groups. Heart rate was slightly decreased during shock and returned to baseline level after resuscitation. No significant differences in MAP and HR were observed among groups throughout the entire experiment (P > 0.05).
Table 1.
Baseline parameters of experimental groups
| Group | I | II | III | IV | V | VI | VII | VIII |
|---|---|---|---|---|---|---|---|---|
| MAP (mmHg) | 122 ± 2 | 122 ± 3 | 119 ± 1 | 113 ± 4 | 116 ± 5 | 116 ± 4 | 112 ± 2 | 112 ± 2 |
| HR (beats/min) | 340 ± 8 | 360 ± 10 | 342 ± 9 | 354 ± 20 | 338 ± 19 | 370 ± 16 | 353 ± 22 | 373 ± 12 |
| A1 blood flow (ng/100 mL) | 51.5 ± 13.5 | 45.4 ± 5.8 | 46.2 ± 9.6 | 55.1 ± 5.7 | 49.8 ± 4.8 | 58.1 ± 12.6 | 43.3 ± 8.3 | 41.7 ± 5.0 |
| A1 diameter (μm) | 82.5 ± 5.2 | 78.5 ± 3.6 | 87.0 ± 1.2 | 85.1 ± 3.7 | 85.7 ± 2.8 | 85.3 ± 5.0 | 76.7 ± 2.1 | 77.2 ± 4.9 |
| A2 diameter (μm) | 25.0 ± 2.5 | 27.7 ± 2.2 | 26.3 ± 2.3 | 27.8 ± 3.1 | 28.5 ± 2.5 | 33.6 ± 1.0 | 28.0 ± 2.5 | 27.7 ± 3.3 |
| pA3 diameter (μm) | 12.6 ± 0.6 | 12.3 ± 0.9 | 12.5 ± 0.6 | 11.4 ± 0.6 | 13.2 ± 0.4 | 12.3 ± 0.3 | 12.2 ± 0.6 | 11.1 ± 0.6 |
| dA3 diameter (μm) | 9.4 ± 0.5 | 8.9 ± 0.9 | 8.8 ± 0.4 | 9.3 ± 0.3 | 10.0 ± 0.2 | 8.5 ± 0.3 | 9.3 ± 0.7 | 8.7 ± 0.3 |
| V1 diameter (μm) | 200.0 ± 10.0 | 200.7 ± 10.2 | 215.0 ± 9.1 | 203.4 ± 15.9 | 213.8 ± 11.3 | 190.0 ± 22.0 | 174.2 ± 7.8 | 192.8 ± 8.9 |
Group I = DPR; group II = CR; group III = DPR + glibenclamide; group IV = DPR + l-NMMA; group V = DPR + glibenclamide + l-NMMA + DPCPX; group VI = DPR + mefenamic acid; group VII = DPR + tetrabutylammonium; and group VIII = CR + BQ123 + BQ788. All data are presented as mean ± SE. There were no significant differences among groups for each parameter by two-way ANOVA and Bonferroni posttests (P > 0.05).
Microvascular response to hemorrhagic shock varied with vascular level, which was consistent with our previous studies. There was a selective vasoconstriction of the larger inflow A1 arterioles (−30% from baseline) and transitional A2 arterioles (−16% from baseline), which was not observed in the smaller A3 premucosal arterioles (Figs. 2 and 3). However, the vasomotion of the A3 arterioles was more frequent and greater during hemorrhagic shock compared with baseline. Hemorrhagic shock caused a significant reduction of blood flow in the A1 arterioles to −65% from baseline in all hemorrhage groups (Fig. 4). In the venous side, hemorrhagic shock caused a significant constriction in the outflow venule V1 that constricted to −30% from baseline in all hemorrhage groups. There were no significant differences in vascular diameter and blood flow changes among groups during hemorrhagic period (P > 0.05).
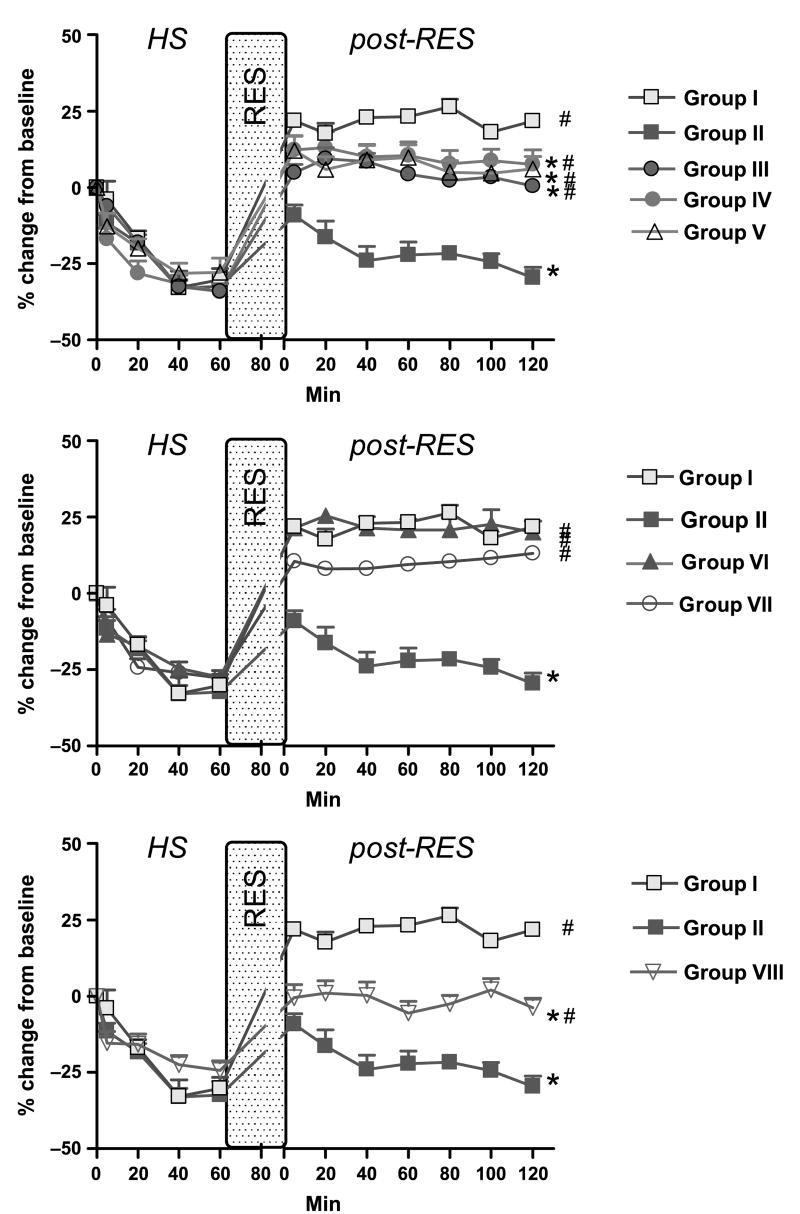
Fig. 2. First-order A1 arteriole diameter data.
Group I = DPR; group II = CR; group III = DPR + glibenclamide; group IV = DPR + l-NMMA; group V = DPR + glibenclamide + l-NMMA + DPCPX; group VI = DPR + mefenamic acid; group VII = DPR + tetrabutylammonium; and group VIII = CR + BQ123 + BQ788. *P < 0.05 vs. DPR, #P < 0.05 vs. CR by two-way ANOVA and Bonferroni posttests. RES indicates resuscitation; HS = Hemorrhagic Shock; RES = Resuscitation.
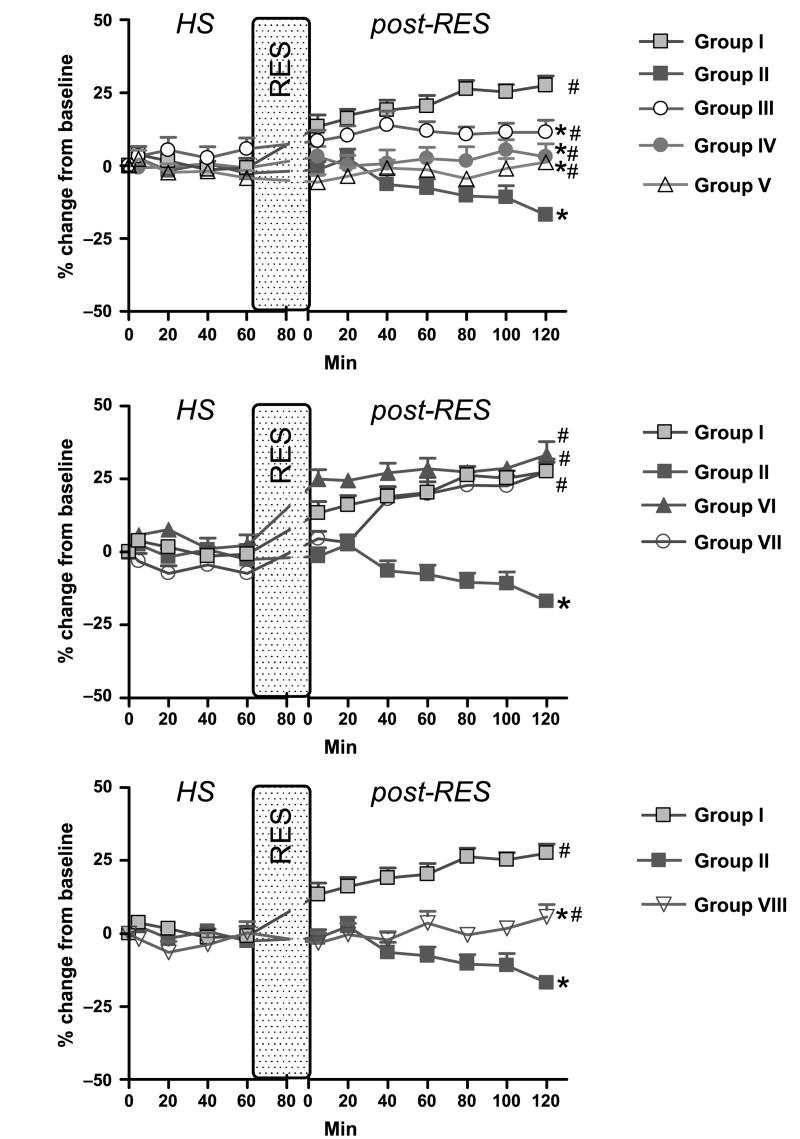
Fig. 3. Distal A3 arteriole diameter data.
Group I = DPR; group II = CR; group III = DPR + glibenclamide; group IV = DPR + l-NMMA; group V = DPR + glibenclamide + l-NMMA + DPCPX; group VI = DPR + mefenamic acid; group VII = DPR + tetrabutylammonium; and group VIII = CR + BQ123 + BQ788. *P < 0.05 vs. DPR, #P < 0.05 vs. CR by two-way ANOVA and Bonferroni posttests. RES indicates resuscitation.
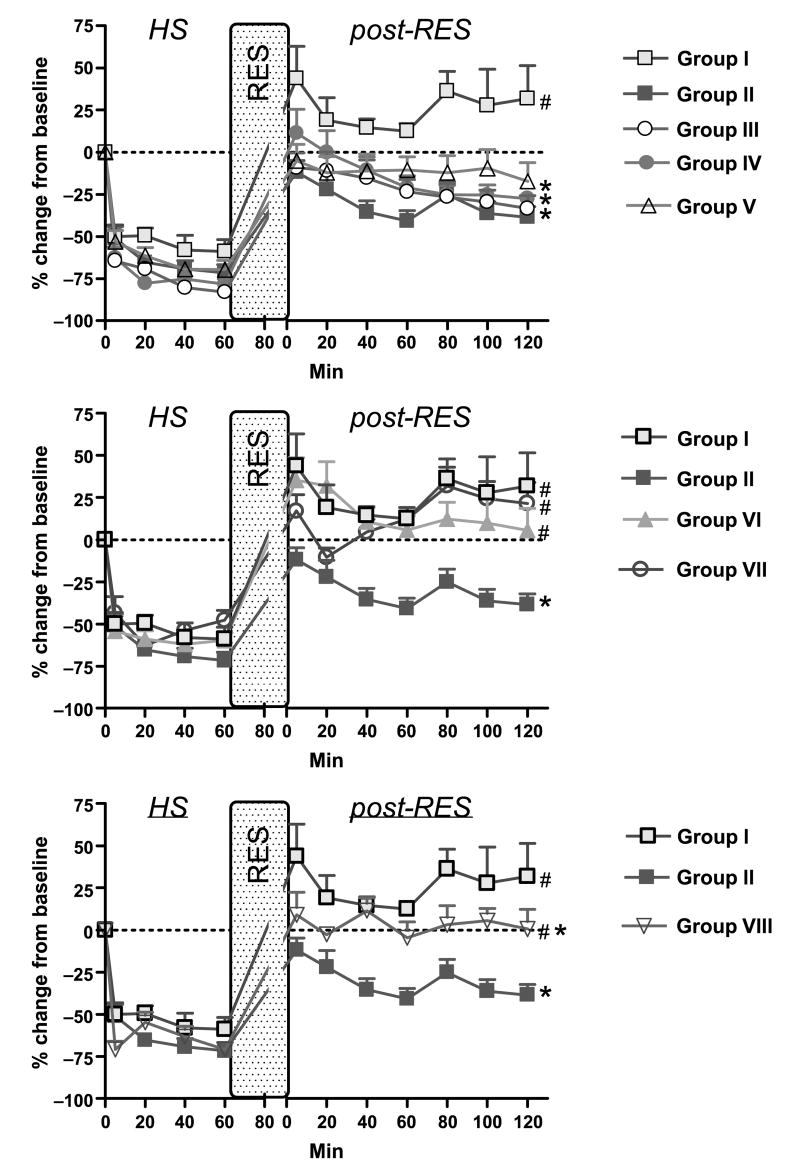
Fig. 4. Blood flow in A1 arteriole data.
Group I = DPR; group II = CR; Group III = DPR + glibenclamide; group IV = DPR + l-NMMA; group V = DPR + glibenclamide + l-NMMA + DPCPX; group VI = DPR + mefenamic acid; group VII = DPR + tetrabutylammonium; and group VIII = CR + BQ123 + BQ788. *P < 0.05 vs. DPR, #P < 0.05 vs. CR by two-way ANOVA and Bonferroni posttests. RES indicates resuscitation.
Conventional resuscitation (group II) initially restored the intestinal microvascular diameters to prehemorrhage level. This was followed by a progressive intestinal microvascular vasoconstriction and hypoperfusion at all microvascular levels during the postresuscitation period (Figs. 3 and 4). In contrast, adjunctive DPR (group I) not only reversed the CR-related postresuscitation vasoconstriction and hypoperfusion, but also induced an instantaneous and sustained vasodilation and hyperperfusion during the entire postresuscitation period at all microvascular levels (A1 arteriole: 21.8% ± 2.4% vs. −29.6% ± 3.5%; distal premucosal third-order (dA3) arteriole: 27.6% ± 3.1% vs. −16.9% ± 2.2% from baseline in group I versus group II).
The effects of inhibitors application on the DPR-mediated postresuscitation intestinal vasodilation are depicted in Figure 3. Topical application of glibenclamide partially attenuated the DPR-induced postresuscitation vasodilation at all intestinal microvascular levels. l-NMMA alone caused a greater reduction of the dilation to the baseline level in all vessels (A1 arteriole: 7.7% ± 4.6% vs. 21.8% ± 2,4%; dA3 arteriole: 3.1% ± 4.5% vs. 27.6% ± 3.1% at 2 h postresuscitation, n = 8; P < 0.05). A1 blood flow at 2 h postresuscitation was also reduced in the presence of l-NMMA (−27.6% ± 4.3% vs. 31.9% ± 19.5%). Combination of l-NMMA, glibenclamide, and DPCPX reduced the postresuscitation DPR-mediated dilation to the baseline level. It did not, however, further constrict the vessels as l-NMMA did alone. Mefenamic acid had no effect on DPR-related vasodilation and blood flow increase during postresuscitation period. Tetrabutylammonium had a minor, if any, role in DPR-induced postresuscitation vasodilation (Fig. 3, middle panel).
To investigate the role of the endothelium-derived endothelin (ET-1) on the progressive intestinal vasoconstriction and hypoperfusion noted after CR from hemorrhagic shock, specific (ET-1) receptors antagonists were topically applied in the tissue bath. As seen in the lower panel of Figure 3, blockade of ET-1 receptors A and B with BQ123 and BQ788 reversed the CR-related intestinal microvascular vasoconstriction (A1 arteriole: −3.7% ± 3.1% vs. −29.6% ± 3.5%; dA3 arteriole: 5.7% ± 4.2% vs. −16.9% ± 2.2% at 2 h postresuscitation, n = 8, P < 0.05) and hypoperfusion (0.6% ± 11.8% vs. −38.6% ± 6.3% at 2 h postresuscitation, n = 8; P < 0.05; Fig. 4, lower panel). V1 diameters and blood outflow returned to baseline and remained at approximately −10% and −25% of baseline, respectively, with or without the presence of inhibitors.
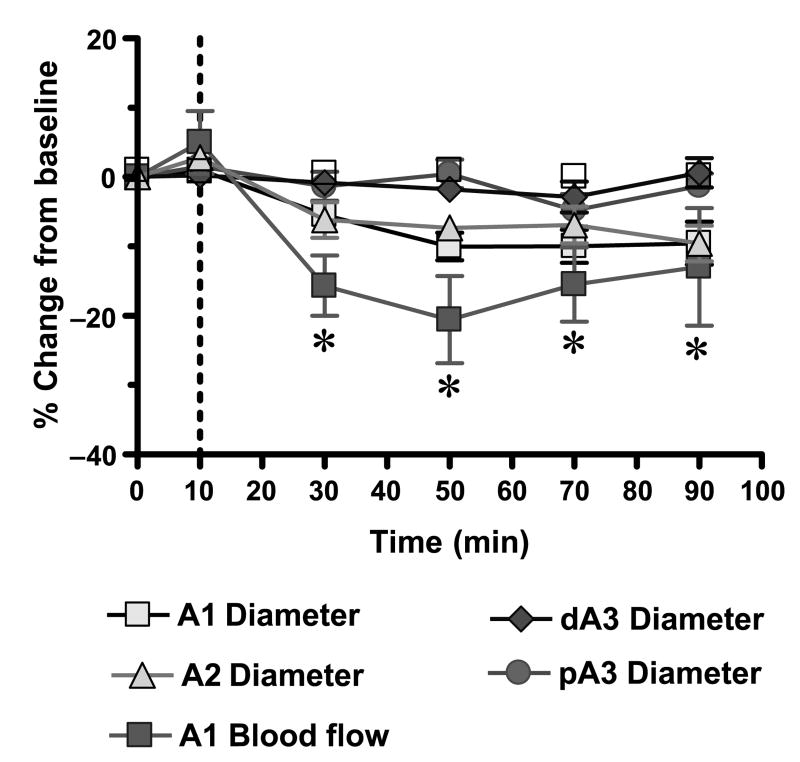
In separate series of control experiments, the intestinal microvascular diameter and flow were assessed after combined blockade of NO, KATP, and adenosine A1 receptors subtype in naive animals. As seen in Figure 5, this combined inhibition in the absence of the vasoactive components of the dialysis solutions produced a marginal vasoconstriction of the inflow A1 and transitional A2 arterioles. This vasoconstriction produced a modest decrease in the A1 blood flow (n = 7, t = 2.599, P < 0.05).
Fig. 5. Effects of inhibition of endothelium-dependent dilation pathways.
*P < 0.05 versus baseline A1 blood flow by Student t test. A1 indicates intestinal inflow first-order arteriole; A2, intestinal second-order arteriole; pA3, proximal premucosal third-order arteriole; dA3, distal premucosal third-order arteriole.
Discussion
The salient findings of the present studies are (1) hemorrhagic shock causes a selective vasoconstriction of the intestinal inflow A1 feed arterioles and spares the smaller precapillary premucosal arterioles, which instead exhibit a marked increase in vasomotion; (2) CR from hemorrhagic shock, which restores and maintains central hemodynamics, causes a persistent and progressive vasoconstriction and hypoperfusion at all intestinal microvascular levels; (3) endothelium-derived ET-1 is an important mediator of the intestinal vasoconstriction and hypoperfusion noted after CR; and (4) DPR reverses the postresuscitation vasoconstriction and hypoperfusion to a state of instantaneous and sustained vasodilation and hyperperfusion by endothelium-dependent mechanisms that involve the NO pathway, the endothelium hyperpolarizing factor pathway, and an adenosine receptor–mediated NO release mechanism.
Rationale for DPR
Regulation of the resting vascular tone down the vascular tree depends largely on vessel size (21). Whereas the control of vascular tone of arteries and arterioles is primarily central via neurohormonal pathways, the vascular tone control of terminal microvessels and precapillary arterioles is primarily local (autoregulation) using metabolic and paracrine changes within the local tissue microenvironment (21, 22). The circulatory collapse caused by blood loss stems from a disparity between the central and the local autoregulatory mechanisms in the face of a progressive splanchnic tissue hypoperfusion (23, 24). Hemorrhagic shock activates baroreceptors-stimulated central compensatory adjustments via neurohormonal stimuli that result in a redistribution of blood flow between and within vital organ systems to increase tissue oxygen demand, decrease systemic oxygen utilization, elevate cardiac output, decrease arteriovenous oxygen gradient, and produce lactic acidosis (25–29). This preferential redistribution of the cardiac output occurs at the expense of other vascular beds such as the gut (30). However, the local autoregulatory adjustments that normally regulate local tissue perfusion exclusively via metabolic and paracrine mechanisms are rendered ineffective during hemorrhagic shock because of either a metabolic defect in tissue oxygen utilization or a microcirculatory defect in the distribution of nutrient blood flow. The erratic blood flow in the precapillary arterioles supports a microcirculatory defect in the distribution of O2 and nutrient blood flow because of the absence of capillary filling during hemorrhage with CR. Although all available resuscitation regimens promptly restore central hemodynamics and compliment the final goal of the central compensatory mechanisms, none of these regimens are known to restore the local microvascular functions, which determine the local tissue perfusion. As demonstrated in the present studies, DPR exerts its maximal vascular effects locally on the small premucosal and the precapillary arterioles that determine the number of perfused capillaries and, hence, the effective vascular surface area available for oxygen and nutrients exchange.
Mechanisms of action of DPR
The microvascular effects of DPR in the present studies are attributed to hyperosmolality, glucose, and the lactate buffer anion system of the peritoneal resuscitation solution (16, 17). These vasoactive components appear to induce intestinal microvascular vasodilation exclusively by endothelium-dependent mechanisms. The magnitude of the DPR-mediated intestinal microvascular vasodilation is similar to the dilation obtained with the clinical peritoneal dialysis solutions in the intestinal microvasculature of naive animals (17). These data suggest that the vasoactive components of the peritoneal solution must have evoked, synergistically acted on, and initialized the same endothelium-dependent dilation pathways both in hemorrhage/resuscitation or naive animals. Generalized endothelial cell dysfunction has been implicated in the microvascular derangements caused by hemorrhagic shock with CR (12). Wang et al. (31) showed that impairment of vascular response to acetylcholine occurs very early after trauma-hemorrhage and persists despite fluid resuscitation. We have reported that shock-induced impairment of the dilation function is exclusively endothelium-dependent and not related to any measurable derangement of the vascular smooth muscle function (32). Such impairment of the endothelium dilation function, at least in part, can be explained by reports noting reduced tissue levels of NO and dilator prostanoids after hemorrhage and CR (33, 34). The marked reduction in DPR-induced vasodilation after NO inhibition in the present studies suggests that DPR preserves an endothelial-derived vasodilator (NO) function and therefore improves vascular reactivity and tissue perfusion after hemorrhagic shock.
The ATP-sensitive potassium channel (KATP) is involved in vascular tone regulation by modulation of cell membrane potential. Hyperosmolality-mediated dilation in muscle arterioles is stimulated by activation of the glibenclamide-sensitive potassium channels KATP (35, 36). Hemorrhagic shock is known to alter membrane fluidity and cell membrane potential (37–39). Studies have shown that intravenous administration of glibenclamide causes a sustained recovery of hemodynamics and tissue perfusion during hemorrhagic shock and inadequate resuscitation (40, 41). In the present studies, topical application of glibenclamide partially attenuated the DPR-related vasodilation and hyperperfusion. This finding indicates that KATP channels are activated during adjunctive DPR and that such activation contributes to the DPR-related vasodilation and hyperperfusion.
Flynn et al. (42) have shown that continuous suffusion of the terminal ileum with an isotonic glucose solution after hemorrhagic shock significantly increases the intestinal feeding arteriolar blood flow presumably by selective submucosal and previllus arteriolar dilation that is likely mediated by locally generated vasodilators. We have shown that topical exposure of the terminal ileum in naive rats to an isotonic glucose solution produces an insidious time-dependent vasodilation preferentially in the smaller intestinal premucosal arterioles (16). This selective vasodilation was caused by NO release via adenosine A1 receptors activation secondary to intercellular glucose uptake (16). These data and the present studies suggest that glucose-based peritoneal dialysis solutions produce an instantaneous hyperosmolality-mediated microvascular relaxation by hyperpolarization secondary to activation of glibenclamide-sensitive K+ channels (KATP) and activation of adenosine A1 receptor-mediated NO release, associated with energy-dependent intracellular glucose uptake. Furthermore, as shown in the present studies, in the absence of the vasoactive components of the dialysis solution, combined inhibition of the NO, the KATP channels, and the adenosine A1 receptor subtypes in naive rats produced a marginal vasoconstriction only in the inflow A1 arterioles, suggesting that the vasoactive components of the dialysis solution are required for these endothelium-dependent mechanisms. Finally, we have recently verified and confirmed these mechanisms in the intestinal vascular bed of naive rats exposed to dialysis solutions (43). It may be argued that the use of the hypertonic peritoneal dialysis solution for DPR might have removed “vasoconstrictors” products accumulated during hemorrhagic shock and resuscitation. This is unlikely to occur given the experimental design of simulated DPR in a small 2-cm segment of the terminal ileum and the instantaneous vasodilator effect of the DPR fluid in the present studies.
Mechanisms of postresuscitation microvascular dysfunction
It is generally conceived that endothelial cell dysfunction is the cause of the postresuscitation microvascular derangements. Such global endothelial cell dysfunction might encompass an imbalance between endothelium-dependent vasodilators and constrictors mechanism. Endothelin (ET-1) is a potent endothelial-derived constrictor peptide that regulates vascular reactivity in a paracrine and remote fashion. ET-1 production is stimulated by reduced wall shear stress and tissue injury (44); both stimuli are prevalent in hemorrhagic shock. Indeed, ET-1 expression and production are up-regulated during hemorrhagic shock in various tissues (45), leading to pronounced mucosal damage, reduction of functional capillary density, and failure of intestinal perfusion (46, 47). These studies indicate that ET-1 is the likely candidate which explains the intestinal microvascular vasoconstriction and hypoperfusion after resuscitation from hemorrhagic shock. This is supported by findings in the present studies showing complete abolition of the progressive postresuscitation vasoconstriction and hypoperfusion with ET-1 antagonists (group VIII). Interestingly, DPR not only abolished the progressive postresuscitation vasoconstriction and hypoperfusion, but also produced an instantaneous and sustained vasodilation and hyperperfusion. This suggests that DPR overrides any prevailing constrictor mechanisms and shifts the balance toward the endothelium-dependent vasodilation mechanisms.
Overall, we conclude that DPR improves splanchnic tissue perfusion by endothelium-dependent mechanisms mediated by activations of glibenclamide-sensitive K+ channels (KATP), adenosine A1 receptor subtype, and NO (NO) release. Direct peritoneal resuscitation preserves endothelial dilatory functions and overrides any endothelium-derived constrictor response triggered by hemorrhagic shock and CR.
Acknowledgments
This project was supported by a VA Merit Review grant and by NIH research grant no. 5R01 HL076160-03, funded by the National Heart, Lung, and Blood Institute and the United States Army Medical Resources and Material Command.
Footnotes
Data from the present studies were presented at the 29th Annual Conference on Shock held in Broomfield, CO, June 3–6, 2006, and published in abstract form in Shock 2006;25 suppl 1.
References
- 1.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. doi: 10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Zakaria ER, Spain DA, Harris PD, Garrison RN. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002;18:567–573. doi: 10.1097/00024382-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Mazzoni MC, Borgstrom P, Intaglietta M, Arfors KE. Lumenal narrowing and endothelial cell swelling in skeletal muscle capillaries during hemorrhagic shock. Circ Shock. 1989;29:27–39. [PubMed] [Google Scholar]
- 5.Scalea TM, Maltz S, Yelon J, Trooskin SZ, Duncan AO, Sclafani SJ. Resuscitation of multiple trauma and head injury: role of crystalloid fluids and inotropes. Crit Care Med. 1994;22:1610–1615. [PubMed] [Google Scholar]
- 6.Abou-Khalil B, Scalea TM, Trooskin SZ, Henry SM, Hitchcock R. Hemodynamic responses to shock in young trauma patients: need for invasive monitoring. Crit Care Med. 1994;22:633–639. doi: 10.1097/00003246-199404000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Abramson D, Scalea TM, Hitchcock R, Trooskin SZ, Henry SM, Greenspan J. Lactate clearance and survival following injury. J Trauma. 1993;35:584–588. doi: 10.1097/00005373-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Broder G, Weil MH. Excess lactate: an index of reversibility of shock in human patients. Science. 1964;143:1457–1459. doi: 10.1126/science.143.3613.1457. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford EJ, Morris JA, Jr, Reed GW, Hall KS. Base deficit stratifies mortality and determines therapy. J Trauma. 1992;33:417–423. doi: 10.1097/00005373-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Dunham CM, Siegel JH, Weireter L, Fabian M, Goodarzi S, Guadalupi P, Gettings L, Linberg SE, Vary TC. Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock. Crit Care Med. 1991;19:231–243. doi: 10.1097/00003246-199102000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Heyland DK, Cook DJ, King D, Kernerman P, Brun-Buisson C. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996;24:517–524. doi: 10.1097/00003246-199603000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. doi: 10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Scalia SV, Taheri PA, Force S, Ozmen V, Lui D, Fish J, Hansen D, Chambers R, Flint L, Steinberg S. Mesenteric microcirculatory changes in nonlethal hemorrhagic shock: the role of resuscitation with balanced electrolyte or hypertonic saline/dextran. J Trauma. 1992;33:321–325. [PubMed] [Google Scholar]
- 14.Zakaria ER, Garrison RN, Spain DA, Matheson PJ, Harris PD, Richardson JD. Intraperitoneal resuscitation improves intestinal blood flow following hemorrhagic shock. Ann Surg. 2003;237:704–711. doi: 10.1097/01.SLA.0000064660.10461.9D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakaria ER, Hurt RT, Matheson PJ, Garrison RN. A novel method of peritoneal resuscitation improves organ perfusion after hemorrhagic shock. Am J Surg. 2003;186:443–448. doi: 10.1016/j.amjsurg.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Zakaria ER, Hunt CM, Li N, Harris PD, Garrison RN. Disparity in osmolarity-induced vascular reactivity. J Am Soc Nephrol. 2005;16:2931–2940. doi: 10.1681/ASN.2004090764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakaria ER, Spain DA, Harris PD, Garrison RN. Generalized dilation of the visceral microvasculature by peritoneal dialysis solutions. Perit Dial Int. 2002;22:593–601. [PubMed] [Google Scholar]
- 18.Zakaria ER, Garrison RN, Kawabe T, Harris PD. Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation. J Trauma. 2005;58:499–506. doi: 10.1097/01.TA.0000152892.24841.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrison RN, Conn AA, Harris PD, Zakaria ER. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2000;136:900–908. doi: 10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Bohlen HG, Gore RW. Preparation of rat intestinal muscle and mucosa for quantitative microcirculatory studies. Microvasc Res. 1976;11:103–110. doi: 10.1016/0026-2862(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 21.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182–196. doi: 10.1006/jsre.2000.5862. [DOI] [PubMed] [Google Scholar]
- 22.Bohlen HG, Hutchins PM, Rapela CE, Green HD. Microvascular control in intestinal mucosa of normal and hemorrhaged rats. Am J Physiol. 1975;229:1159–1164. doi: 10.1152/ajplegacy.1975.229.5.1159. [DOI] [PubMed] [Google Scholar]
- 23.Zweifach BW. Mechanisms of blood flow and fluid exchange in microvessels: hemorrhagic hypotension model. Anesthesiology. 1974;41:157–168. doi: 10.1097/00000542-197408000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Zweifach BW, Fronek A. The interplay of central and peripheral factors in irreversible hemorrhagic shock. Prog Cardiovasc Dis. 1975;18:147–180. doi: 10.1016/0033-0620(75)90003-1. [DOI] [PubMed] [Google Scholar]
- 25.Reilly PM, Bulkley GB. Vasoactive mediators and splanchnic perfusion. Crit Care Med. 1993;21:55–68. doi: 10.1097/00003246-199302001-00011. [DOI] [PubMed] [Google Scholar]
- 26.Toung T, Reilly PM, Fuh KC, Ferris R, Bulkley GB. Mesenteric vasoconstriction in response to hemorrhagic shock. Shock. 2000;13:267–273. doi: 10.1097/00024382-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Hollenberg NK, Nickerson M. Changes in pre-and postcapillary resistance in pathogenesis of hemorrhagic shock. Am J Physiol. 1970;219:1483–1489. doi: 10.1152/ajplegacy.1970.219.5.1483. [DOI] [PubMed] [Google Scholar]
- 28.Neutze JM, Wyler F, Rudolph AM. Changes in distribution of cardiac output after hemorrhage in rabbits. Am J Physiol. 1968;215:857–864. doi: 10.1152/ajplegacy.1968.215.4.857. [DOI] [PubMed] [Google Scholar]
- 29.Kovach AG, Rosell S, Sandor P, Koltay E, Kovach E, Tomka N. Blood flow, oxygen consumption, and free fatty acid release in subcutaneous adipose tissue during hemorrhagic shock in control and phenoxybenzamine-treated dogs. Circ Res. 1970;26:733–741. doi: 10.1161/01.res.26.6.733. [DOI] [PubMed] [Google Scholar]
- 30.Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15:329–343. doi: 10.1097/00024382-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Ba ZF, Chaudry IH. Endothelial cell dysfunction occurs very early following trauma-hemorrhage and persists despite fluid resuscitation. Am J Physiol. 1993;265:973–979. doi: 10.1152/ajpheart.1993.265.3.H973. [DOI] [PubMed] [Google Scholar]
- 32.Zakaria ER, Garrison RN, Spain DA, Harris PD. Impairment of endothelium-dependent dilation response after resuscitation from hemorrhagic shock involved postreceptor mechanisms. Shock. 2004;21:175–181. doi: 10.1097/00024382-200402000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Fruchterman TM, Spain DA, Matheson PJ, Martin AW, Wilson MA, Harris PD, Garrison RN. Small intestinal production of nitric oxide is decreased following resuscitated hemorrhage. J Surg Res. 1998;80:102–109. doi: 10.1006/jsre.1998.5421. [DOI] [PubMed] [Google Scholar]
- 34.Myers SI, Small J. Prolonged hemorrhagic shock decreases splanchnic prostacyclin synthesis. J Surg Res. 1991;50:417–420. doi: 10.1016/0022-4804(91)90017-g. [DOI] [PubMed] [Google Scholar]
- 35.Ishizaka H, Kuo L. Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. Am J Physiol. 1997;273:H104–H112. doi: 10.1152/ajpheart.1997.273.1.H104. [DOI] [PubMed] [Google Scholar]
- 36.Massett MP, Koller A, Kaley G. Hyperosmolality dilates rat skeletal muscle arterioles: role of endothelial K(ATP) channels and daily exercise. J Appl Physiol. 2000;89:2227–2234. doi: 10.1152/jappl.2000.89.6.2227. [DOI] [PubMed] [Google Scholar]
- 37.Shires GT, Cunningham JN, Backer CR, Reeder SF, Illner H, Wagner IY, Maher J. Alterations in cellular membrane function during hemorrhagic shock in primates. Ann Surg. 1972;176:288–295. doi: 10.1097/00000658-197209000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trunkey DD, Illner H, Wagner IY, Shires GT. The effect of hemorrhagic shock on intracellular muscle action potentials in the primate. Surgery. 1973;74:241–250. [PubMed] [Google Scholar]
- 39.Trunkey DD, Illner H, Arango A, Holiday R, Shires GT. Changes in cell membrane function following shock and cross-perfusion. Surg Forum. 1974;25:1–3. [PubMed] [Google Scholar]
- 40.Maybauer DM, Salsbury JR, Westphal M, Maybauer MO, Salzman AL, Szabo C, Westphal-Varghese BB, Traber LD, Traber DL. The ATP-sensitive potassium-channel inhibitor glibenclamide improves outcome in an ovine model of hemorrhagic shock. Shock. 2004;22:387–391. doi: 10.1097/01.shk.0000140661.78744.f6. [DOI] [PubMed] [Google Scholar]
- 41.Salzman AL, Vromen A, Denenberg A, Szabo C. K(ATP)-channel inhibition improves hemodynamics and cellular energetics in hemorrhagic shock. Am J Physiol. 1997;272:H688–H694. doi: 10.1152/ajpheart.1997.272.2.H688. [DOI] [PubMed] [Google Scholar]
- 42.Flynn WJ, Jr, Gosche JR, Garrison RN. Intestinal blood flow is restored with glutamine or glucose suffusion after hemorrhage. J Surg Res. 1992;52:499–504. doi: 10.1016/0022-4804(92)90318-t. [DOI] [PubMed] [Google Scholar]
- 43.Zakaria ER, Patel AM, Garrison RN. Molecular mechanisms of peritoneal dialysis solution-mediated vasodilation [abstract] Perit Dial Int. 2006;26:S7. [Google Scholar]
- 44.Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989;161:859–864. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]
- 45.Liu LM, Dubick MA. Hemorrhagic shock–induced vascular hyporeactivity in the rat: relationship to gene expression of nitric oxide synthase, endothelin-1, and select cytokines in corresponding organs. J Surg Res. 2005;125:128–136. doi: 10.1016/j.jss.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Massberg S, Boros M, Leiderer R, Baranyi L, Okada H, Messmer K. Endothelin (ET)-1 induced mucosal damage in the rat small intestine: role of ET(A) receptors. Shock. 1998;9:177–183. doi: 10.1097/00024382-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Ba ZF, Shimizu T, Szalay L, Bland KI, Chaudry IH. Gender differences in small intestinal perfusion following trauma hemorrhage: the role of endothelin-1. Am J Physiol Gastrointest Liver Physiol. 2005;288:G860–G865. doi: 10.1152/ajpgi.00437.2004. [DOI] [PubMed] [Google Scholar]