Figure 2.
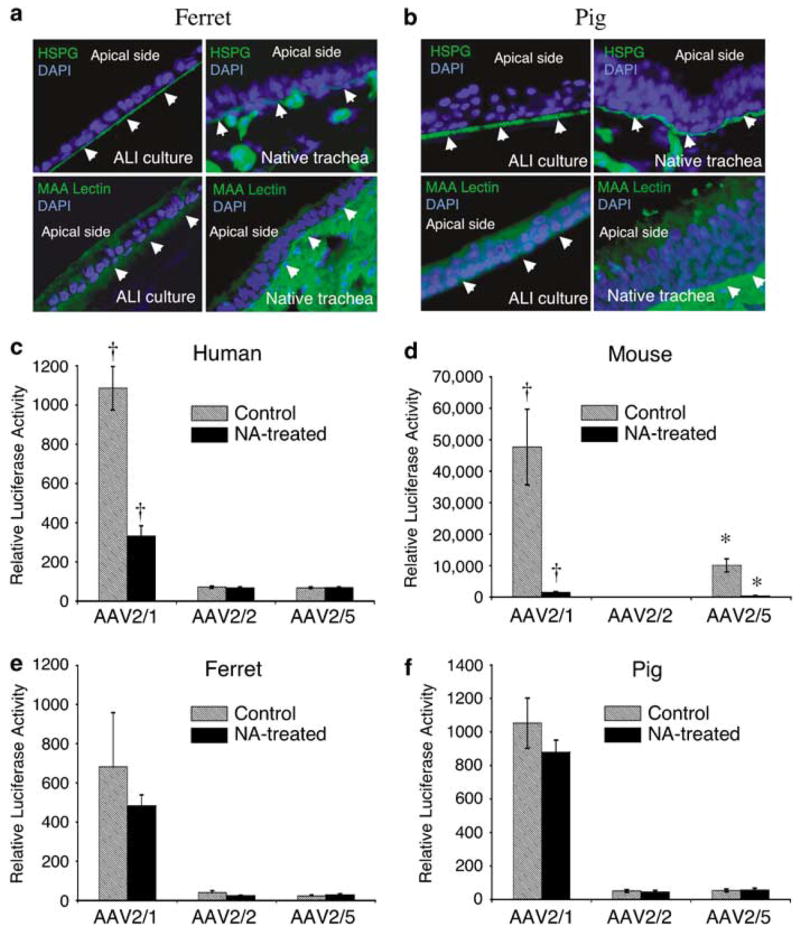
Comparative analysis of candidate viral-binding receptors in human, mouse, ferret and pig airway epithelia. (a, b) Immunostaining for heparan sulfate proteoglycan (HSPG) and 2,3-linked sialic acid using the Maackia amurensis (MAA) lectin on sections from ferret and pig airway epithelial ALI cultures (left panel) and native tracheas (right panel), as described previously for human and mouse ALI cultures and tracheal tissue.11 Arrowheads mark the basolateral side of each epithelium and 4’,6-diamidino-2-phenylindole demarcates nuclei. Antibodies used included rat anti-HSPG monoclonal antibody followed by fluoroisothiocyanate (FITC)-labeled anti-rat secondary antibody, and FITC-labeled MAA lectin. (c–f) ALI epithelial cultures from human, mouse, ferret and pig were pretreated with neuraminidase (NA-treated) to remove sialic acid from the apical surfaces of airway epithelial cultures, or with Ultraser G (USG) medium as a control (untreated), prior to infection with virus. Specifically, 200 μl of either USG medium containing 50 mU ml−1 of type III NA from Vibrio cholerae (Sigma, Santa Louis, MO, USA), or USG medium alone, was added to the apical side of the cultures and left there for 2 h (at 37 °C). NA was then removed and cultures were washed three times with phosphate-buffered saline. This was followed by apical infection at a multiplicity of infection of 2.0 × 103 particles per cell (2 × 109 total particles per well) with the indicated serotypes recombinant adeno-associated virus luciferase virus for 1 h at 4 °C. Cultures were then shifted to a 37 °C incubator and the efficiency of transduction was evaluated by measuring luciferase activity at 7 days post-infection. Data represent the means (± s.e.m.) of the relative luciferase activity (per well) from three independent experiments (N = 12). †,* marked comparisons indicate significant difference as determined by the student’s t-test.
