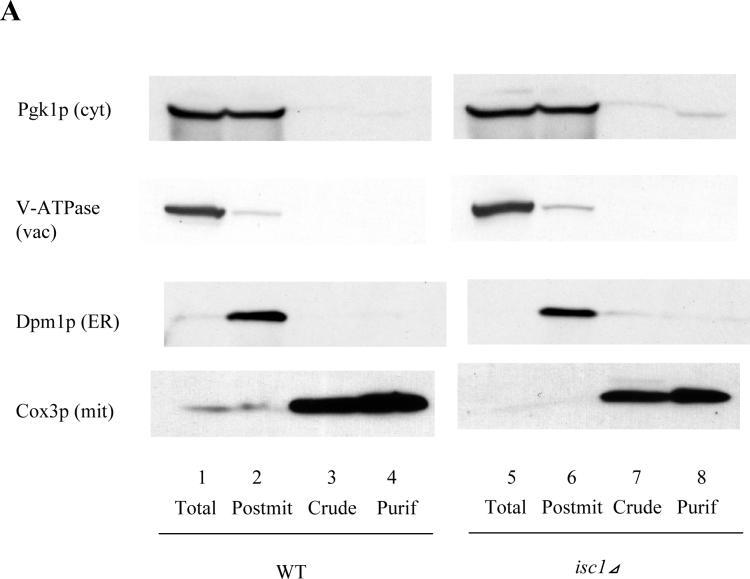
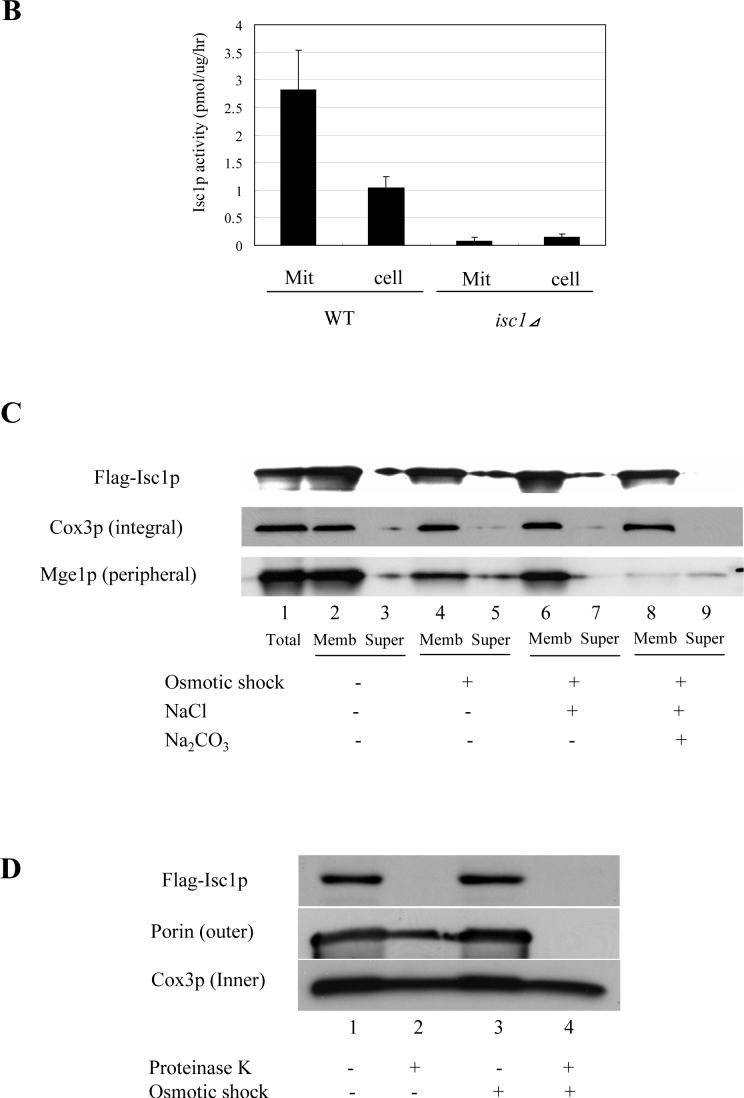
Fig. 1. Purification of mitochondria and submitochondrial fractionation of Flag-Isc1p.
(A) Validation of the purity of mitochondria. Forty micrograms of each sample (lanes 1,5; total cell lysate, lanes 2,6; post-mitochondrial fraction, lanes 3,7; crude mitochondria and lanes 4,8; purified mitochondria) from WT (lanes 1−4) and isc1⊿ (lanes 5−8) were subjected to western blotting against a cytosolic protein, Pgk1p, a vacuolar protein, V-ATPase, an ER membrane protein, Dpm1p, and a mitochondrial membrane protein, Cox3p. (B) Isc1p activity of purified mitochondria. Isc1p activity of purified mitochondria and the whole cell from WT and isc1⊿ was measured basically as previously described (24). (C) Membrane association of Flag-Isc1p. Mitochondria purified from isc1 ⊿+pYES/FLAG-ISC1 were treated without osmotic shock (lanes 2, 3; 250 mM sucrose, 10 mM HEPES, pH 7.2, 1 mM EDTA), with osmotic shock (lanes 4, 5; 10 mM HEPES, pH 7.2, 1 mM EDTA), with osmotic shock and high salt (lanes 6, 7; 0.1 M NaCl, 10 mM HEPES, pH 7.2, 1 mM EDTA), or with osmotic shock and alkali pH (lanes 8, 9; 0.1 M Na2CO3, pH 11.2, 10 mM HEPES, pH 7.2, 1 mM EDTA), centrifuged at 100,000 g for 10 min, and total (lane 1), membrane (lanes 2, 4, 6, 8), and supernatant (lanes 3, 5, 7, 9) were analyzed by western blotting using an antibody against Flag. (D) Submitochondrial localization of Flag-Isc1p. Mitochondria purified from isc1⊿+pYES/FLAG-ISC1 were treated without osmotic shock (lanes 1, 2; 250 mM sucrose, 10 mM HEPES, pH 7.2, 1 mM EDTA) or with osmotic shock (lanes 3, 4; 10 mM HEPES, pH 7.2, 1 mM EDTA) for 10 min on ice and with (lanes 2, 4) or without (lanes 1, 3) final 50 μg/ml proteinase K for 30 min on ice. 2 mM phenylmethylsulfonyl fluoride was added, mitochondria were centrifuged at 12,000 g for 5 min, washed once, and pelles were applied to western analysis with antibody against Flag, porin, or Cox3p.