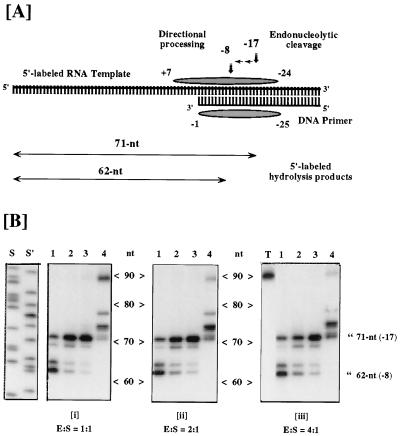
Figure 2.
RNase H activity of selectively deleted RTs. (A) Substrate for RNase H activity is a 90-nt 5′-end labeled RNA hybridized at its 3′ terminus to a 36-nt DNA. Shaded ellipsoids represent template and primer nucleotides covered by HIV-1 RT located over the primer 3′ OH. In the absence of DNA synthesis, endoribonuclease activity ≈17 nt distal to the primer terminus produces a radiolabeled 71-nt product. Subsequent to this, a directional processing activity hydrolyzes in a 3′ → 5′ direction as far as template nucleotide −8 (62-nt product), after which the fragmented template presumably dissociates from primer. (B) RNase H activity of HIV-1 RT derivatives harboring p51 deletions. The ratio of RT to template-primer is indicated below each panel. Lanes: 1, wild-type p66/p51; 2, p66/p51Δ5; 3, p66/p51Δ9; 4, p66/p51Δ13; S and S′, DNA sequence ladders for molecular weight determination; T, no enzyme. Migration positions of the −17 and −8 hydrolysis products are indicated.