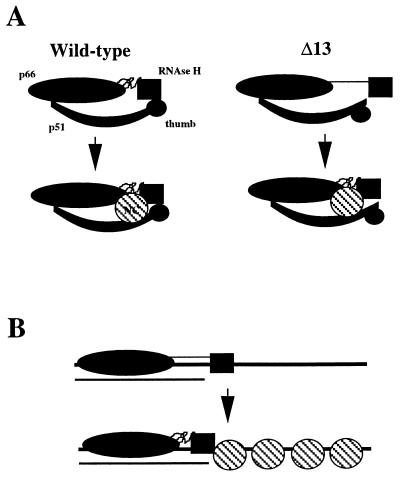
Figure 6.
Models for the suppression of the p66/p51Δ13 defect by HIV NC. (A) Specific Interaction between RT and NC. (Wild-type) The RNase H domain of the wild-type enzyme is stabilized by interactions with the p51 thumb, and this interaction is strengthened by the presence of NC which may interact with p66, the RNase H domain and p51. (Δ13) Deletion of the carboxyl-terminal residues of p51 alters the conformation of the p51 thumb, thus increasing the spatial relationship between the polymerase and RNase H catalytic centers. The wild-type conformation is induced into the p66/p51Δ13 RT in the presence of NC. (B) Nonspecific interaction between RT and NC. For clarity only the large subunit of the p66/p51Δ13 heterodimer is indicated. The nucleic acid that is illustrated represents strong-stop DNA (thick line) with residual template RNA (thin line) bound to the 3′ end of the DNA. The binding of p66/p51Δ13 RT to nucleic acid is dictated by the polymerase domain. The availability of nucleic acid for binding to the RNase H domain 26–27 nt from the polymerase active site permits stable binding of this enzyme to nucleic acid. In the presence of NC, the nucleic acid-binding domain of RNase H is restricted to interaction with the duplex region. In this case, the wild-type conformation is induced solely by the interaction of the RNase H domain with nucleic acid.