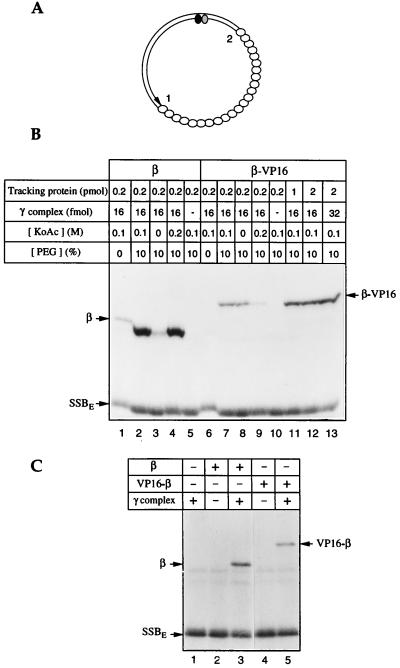
Figure 1.
β–VP16 and VP16–β can be loaded onto DNA. β–VP16 and VP16–β fuse a transcriptional activation domain of VP16 (24) and affinity tags to the C and N ends of E. coli β, respectively. DNA loading and tracking were assessed by photochemical cross-linking (18, 25). (A) The photochemical probe contains a single residue of 5-[N′-(p-azidobenzoyl)-3-aminoallyl]-dUMP (ABdUMP) (shaded ellipse) next to the single radioactive nucleotide ([α-32P]dGMP) (filled ellipse) hundreds of base pairs from a preferred DNA-loading site 1 (a primer-template double strand–single strand junction) and ≈130 bp from a less efficient loading site 2 (the double strand–single strand junction of opposite polarity). The empty ellipses represent E. coli single-stranded DNA binding protein (SSBE) coating the single-stranded portion of the photoactive probe. (B) Loading β-VP16. Proteins, in quantities shown above each lane, were incubated with the photoactive DNA for 15 min at 37°C, in reaction buffer containing 250 μM dATP, and the specified concentrations of potassium acetate and polyethylene glycol. Tracking along DNA from the loading site to the vicinity of the photoactive nucleotide was detected by UV-irradiation, digestion of the resulting covalent protein-DNA adducts with nucleases, and resolution by SDS/PAGE, as described in ref. 25. (C) Loading of VP16–β. Tracking proteins (0.5 pmol of β or VP16–β) and/or γ complex (16 fmol) were incubated with the photoactive DNA for 15 min at 37°C, in reaction buffer containing 100 mM potassium acetate and 250 μM dATP. Samples were irradiated and processed as indicated above.