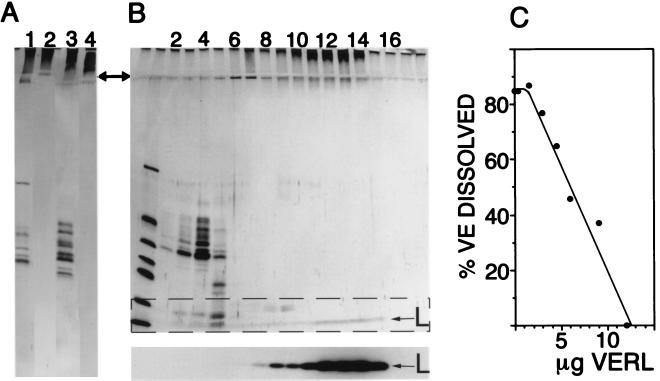
Figure 1.
Identification of VERL. (A) Affinity purification of VERL from two abalone species (Hc and Hr). Lanes: 1, total pH 2.8-soluble VE material (Hc); 2, material eluted by pH 2.8 glycine from the Hc lysin column; 3, starting material from Hr VEs; 4, pH 2.8-eluted material from the Hr lysin column. Material eluted from lysin columns by low pH does not enter the 12% separating gel (arrow notes interface between stacking and separating gels). (B) Sucrose density gradient sedimentation of lysin bound to VERL. (Upper) Silver-stained SDS/12% polyacrylamide gel with the gradient fractions from top (light) to bottom (heavy) going left to right. Lanes: 1, molecular mass standards (13–67 kDa); 2–5, VE components ranging from 30–50 kDa; 9–14, high molecular mass VE material not entering the separating gel. (Lower) Autoradiogram of the dashed rectangle in Upper: 125I-lysin (L) comigrates with the material that does not enter the separating gel. In the absence of VE material, 125I-lysin remained in the top two fractions (not shown). (C) Isolated VERL inhibits the ability of lysin to dissolve isolated VEs. Fifty percent inhibition occurred at a lysin:VERL molar ratio of 69:1.