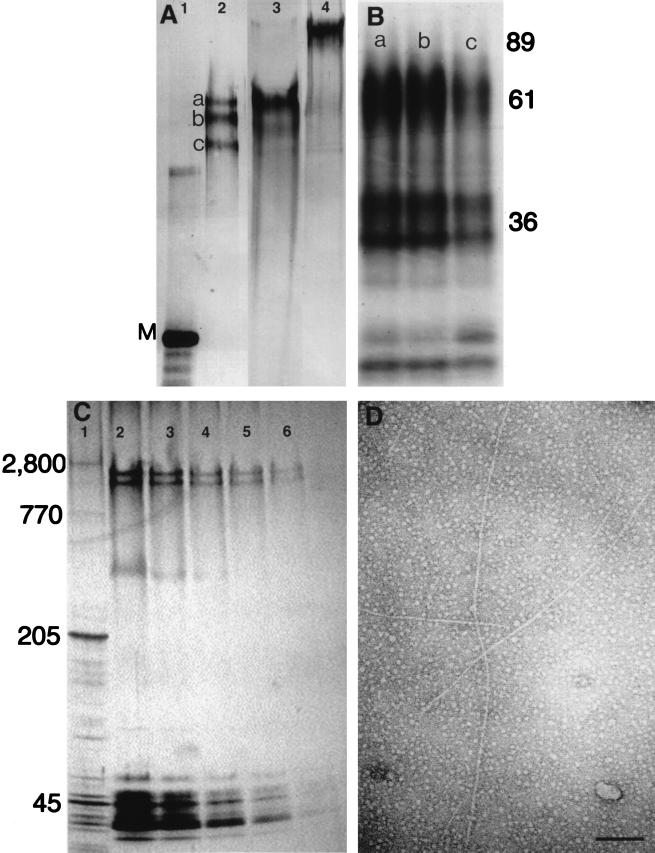
Figure 2.
Characterization of VERL purified by lysin–affinity chromatography. (A) Silver-stained SDS/2.5% polyacrylamide gel. Lanes: 1, rabbit myosin (M; 205 kDa); 2, Hc VERL resolved as three components (a–c) under reducing conditions; 3, Hr VERL resolved as one component under reducing conditions; 4, Hr VERL under nonreducing conditions. (B) Autoradiogram of a SDS/12% polyacrylamide gel of Endoproteinase Glu-C-generated one-dimensional peptide maps of the three Hc VERL bands shown in A (a–c). Positions of molecular mass standards shown on right. (C) A SDS/2.5–15% polyacrylamide gradient gel of isolated Hr VEs quantitatively silver stained (23) and used for densitometry. Lanes: 1, rabbit muscle acetone powder with titin (2,800 kDa) and nebulin (770 kDa) as relative mass standards; 2–6, 50% dilutions of Hr VEs. VERL resolves as two bands below titin. Densitometry of these lanes showed that VERL was approximately 30% of the protein silver-staining material. (D) Isolated Hr VERL negatively stained with uranyl acetate and viewed by transmission electron microscopy. The VERL rods are approximately 13 nm in diameter (7). (Scale bar = 100 nm.)