Figure 3.
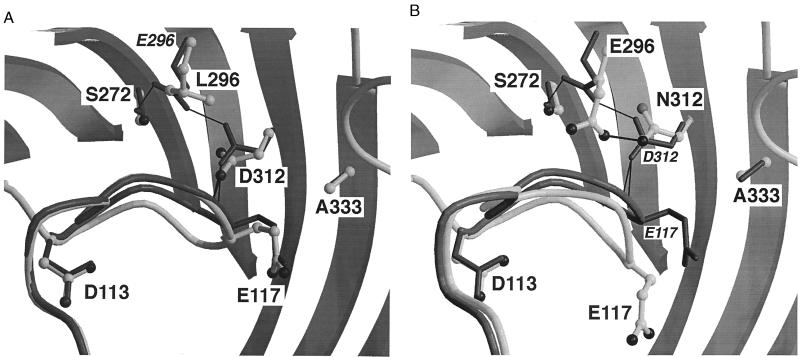
Structure of the L3–barrel interface in OmpF porin mutants E296L (A) and D312N (B). For clarity, only the backbone of the constriction loop and the hydrogen bond network connecting L3 to the barrel are shown. Mutant structures are shown in gray ball-and-stick representation (residues labeled in boldface), with the superimposed wild-type porin structure shown as a black stick-model (residues labeled in italics). Potential hydrogen bonds are shown in thick (mutants) and thin (wild type) lines. The position of L3 is little affected by the E296L mutation (distance between the positions of wild-type and mutant Cα117 = 0.7 Å), and the hydrogen bonds between Asp-312 and the backbone of L3 are conserved. In mutant D312N, L3 has moved toward the channel lumen by 2.5 Å and is partly detached from the barrel wall. Note that the side chain of Glu-117 is not defined by electron density.