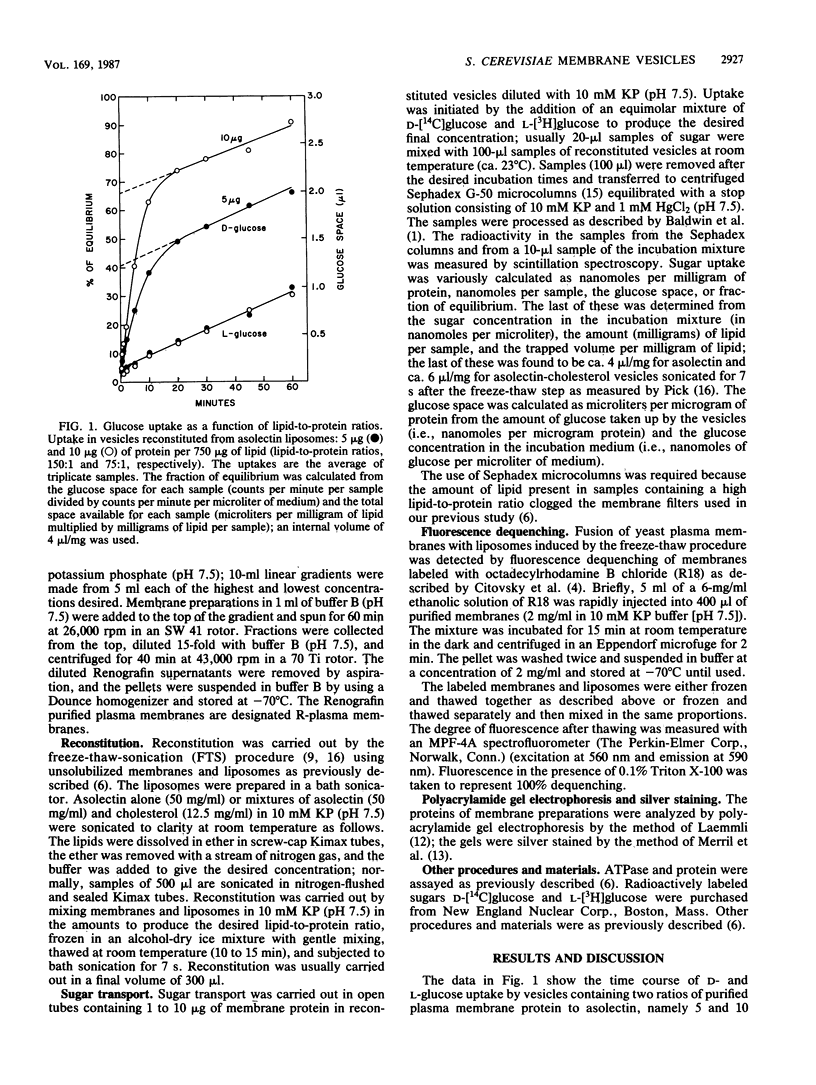
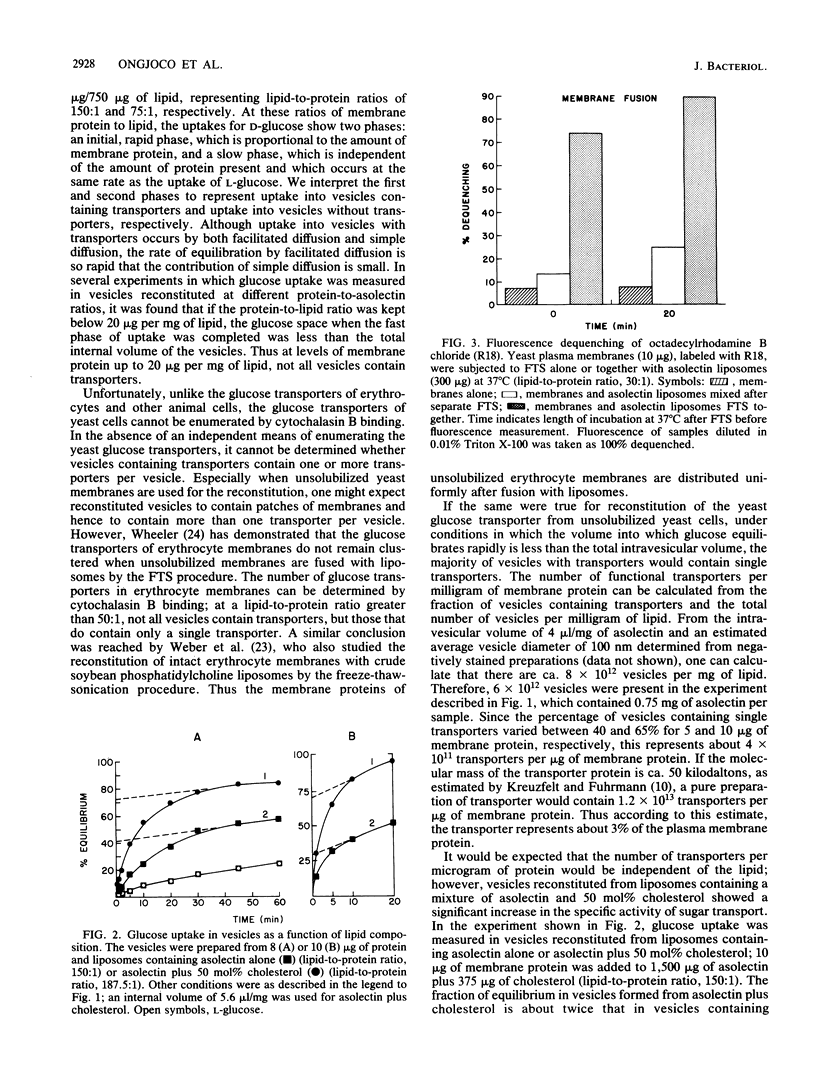
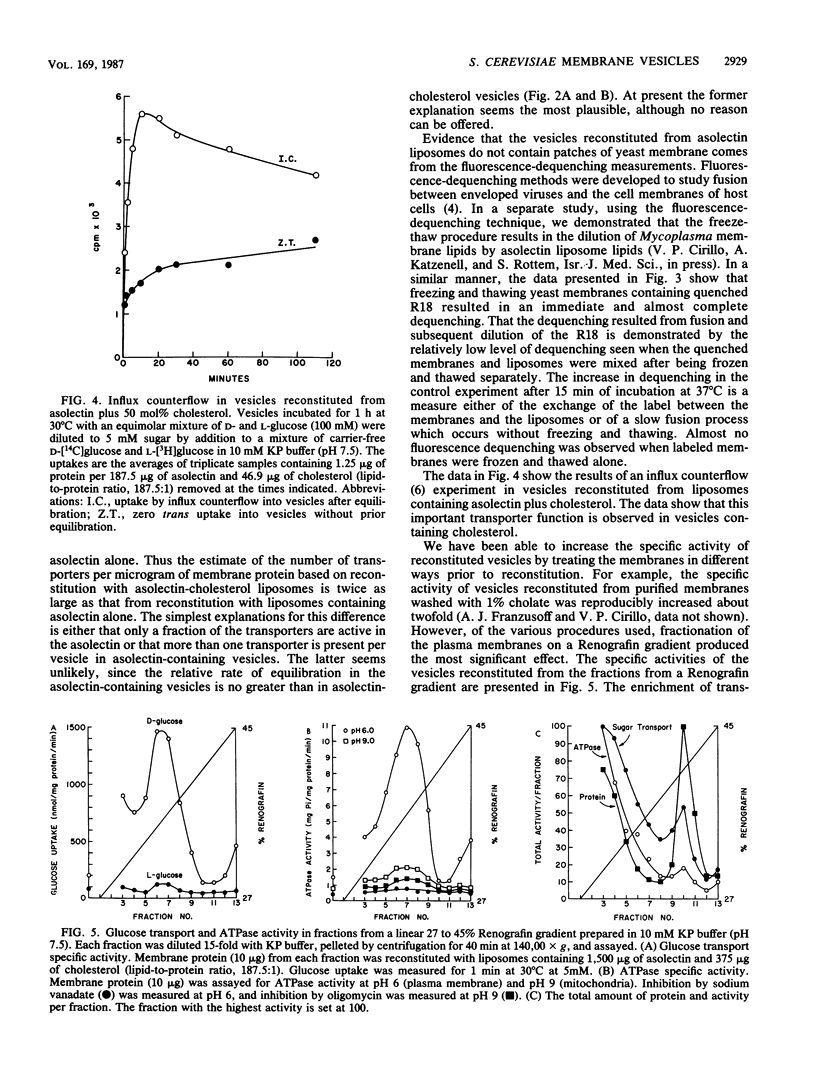
Abstract
Glucose transport activity was reconstituted into liposomes by the freeze-thaw-sonication procedure from unextracted Saccharomyces cerevisiae membranes and preformed phospholipid liposomes. Fluorescence-dequenching measurements with octadecylrhodamine B chloride (R18)-labeled membranes showed that the yeast membrane lipids are diluted by the liposome lipids after the freeze-thaw-sonication procedure. At lipid-to-protein ratios greater than 75:1, vesicles with single transporters were formed. Reconstituted specific activity was increased at least twofold if the liposomes contained 50 mol% cholesterol. A further increase in specific activity, from 3- to 10-fold, was achieved by fractionation of the membranes on a Renografin gradient before reconstitution. Examination of the fractions from the Renografin gradient by sodium dodecyl sulfate-gel electrophoresis showed a parallel enrichment of glucose transport activity and a number of proteins including one with an apparent Mr of ca. 60,000, which might be the glucose transporter. Finally, preliminary kinetic analysis of glucose transport activity in vesicles reconstituted at a high lipid-to-protein ratio gave a Vmax of ca. 2.8 mumol/mg of protein per min at 23 degrees C and a Km of ca. 8 mM. The latter value corresponds to the kinase-independent, low-affinity component of glucose transport observed in wild-type cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M., Gorga J. C., Lienhard G. E. The monosaccharide transporter of the human erythrocyte. Transport activity upon reconstitution. J Biol Chem. 1981 Apr 25;256(8):3685–3689. [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Neigeborn L., Carlson M., Fraenkel D. G. The SNF3 gene is required for high-affinity glucose transport in Saccharomyces cerevisiae. J Bacteriol. 1987 Apr;169(4):1656–1662. doi: 10.1128/jb.169.4.1656-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Blumenthal R., Loyter A. Fusion of Sendai virions with phosphatidylcholine-cholesterol liposomes reflects the viral activity required for fusion with biological membranes. FEBS Lett. 1985 Dec 2;193(2):135–140. doi: 10.1016/0014-5793(85)80137-x. [DOI] [PubMed] [Google Scholar]
- Franzusoff A. J., Cirillo V. P. Glucose transport activity in isolated plasma membrane vesicles from Saccharomyces cerevisiae. J Biol Chem. 1983 Mar 25;258(6):3608–3614. [PubMed] [Google Scholar]
- Franzusoff A., Cirillo V. P. Uptake and phosphorylation of 2-deoxy-D-glucose by wild-type and single-kinase strains of Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Jun 14;688(2):295–304. doi: 10.1016/0005-2736(82)90340-6. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Boehm C., Theuvenet A. P. Sugar transport and potassium permeability in yeast plasma membrane vesicles. Biochim Biophys Acta. 1976 May 21;433(3):583–596. doi: 10.1016/0005-2736(76)90283-2. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kuo S. C., Cirillo V. P. Galactose transport in Saccharomyces cerevisiae. 3. Characteristics of galactose uptake in transferaseless cells: evidence against transport-associated phosphorylation. J Bacteriol. 1970 Sep;103(3):679–685. doi: 10.1128/jb.103.3.679-685.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Serrano R. Purification of the yeast plasma membrane ATPase solubilized with a novel zwitterionic detergent. FEBS Lett. 1980 Feb 25;111(1):69–72. doi: 10.1016/0014-5793(80)80763-0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Pick U. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch Biochem Biophys. 1981 Nov;212(1):186–194. doi: 10.1016/0003-9861(81)90358-1. [DOI] [PubMed] [Google Scholar]
- Serrano R., Delafuente G. Regulatory properties of the constitutive hexose transport in Saccharomyces cerevisiae. Mol Cell Biochem. 1974 Dec 20;5(3):161–171. doi: 10.1007/BF01731379. [DOI] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J. F., Emr S. D., Field C., Schekman R. GAL2 codes for a membrane-bound subunit of the galactose permease in Saccharomyces cerevisiae. J Bacteriol. 1986 Apr;166(1):313–318. doi: 10.1128/jb.166.1.313-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Schekman R. Two distinct subfractions in isolated Saccharomyces cerevisiae plasma membranes. J Bacteriol. 1983 Oct;156(1):222–229. doi: 10.1128/jb.156.1.222-229.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Warden D. A., Semenza G., Diedrich D. F. Solubilization, reconstitution, and attempted affinity chromatography of the sugar transporter of the erythrocyte membrane. J Cell Biochem. 1985;27(2):83–96. doi: 10.1002/jcb.240270203. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J. Reconstitution of glucose transport activity from erythrocyte membranes without detergent and its use in studying effects of ATP depletion. Biochim Biophys Acta. 1986 Jul 24;859(2):180–188. doi: 10.1016/0005-2736(86)90213-0. [DOI] [PubMed] [Google Scholar]