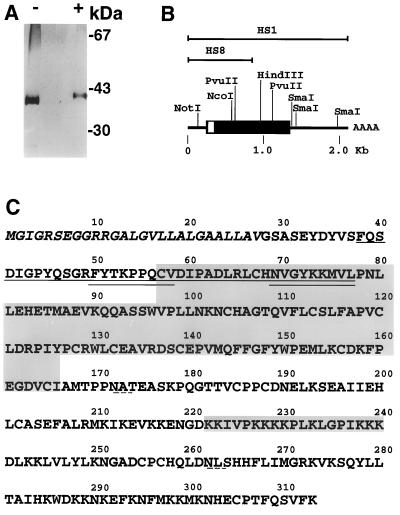
Figure 1.
(A) SDS/PAGE analysis of heparin-Sepharose purified FRP. Approximately 200 ng of protein was resolved in a 4–20% polyacrylamide minigel (Novex) under reducing (+) or nonreducing (−) conditions, and subsequently stained with silver. The position of molecular mass markers is indicated at the right. (B) Representation of human FRP cDNA clones. Overlapping clones HS1 and HS8 are shown above a diagram of the complete coding sequence and the adjacent 5′ and 3′ untranslated regions. The coding region is boxed; the open portion corresponds to the signal sequence. Untranslated regions are represented by a line. Selected restriction sites are indicated. (C) Predicted FRP amino acid sequence (standard single-letter code). The peptide sequence obtained from the purified protein is underlined. Double-underlined sequences were used to generate oligonucleotide probes for screening of the M426 cDNA library. The putative signal sequence is italicized. The large shaded region is the cysteine-rich domain homologous to CRDs in members of the FZ family. The small shaded region is a lysine-rich segment that fulfills the criteria for a consensus hyaluronic acid-binding sequence. The dashed underlining denotes two potential asparagine-linked glycosylation sites.