Abstract
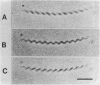
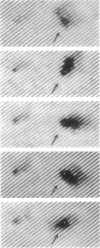
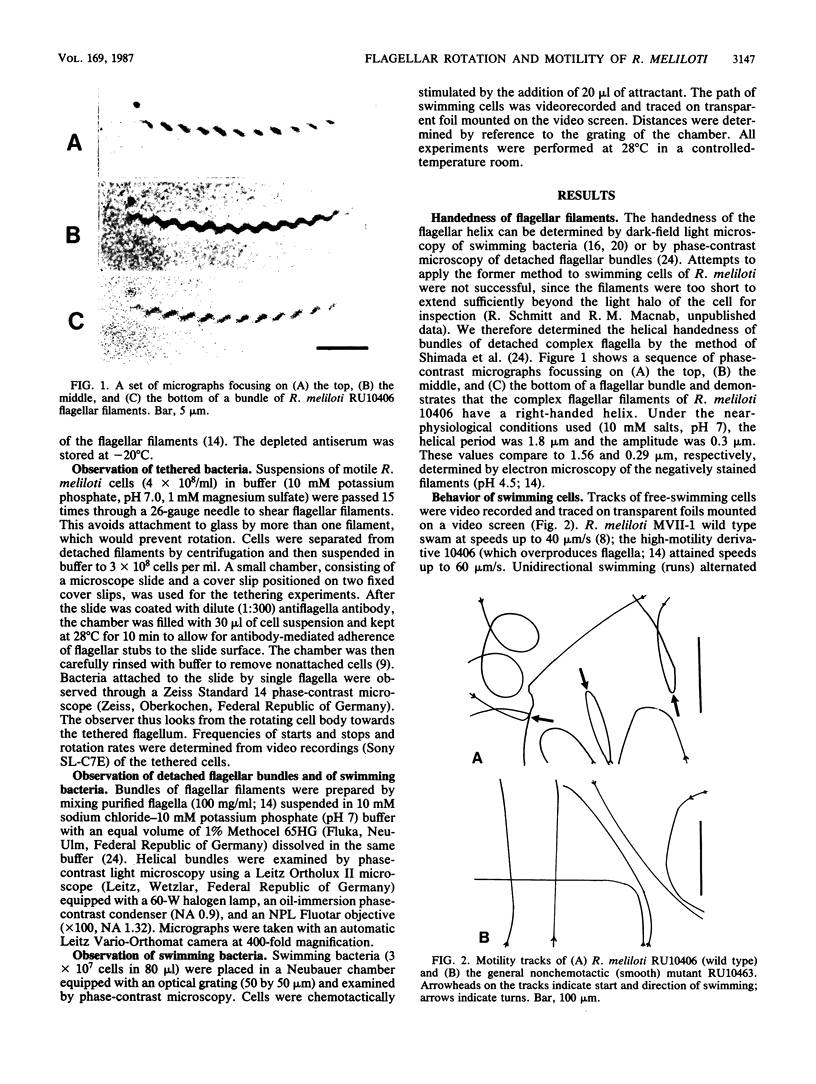
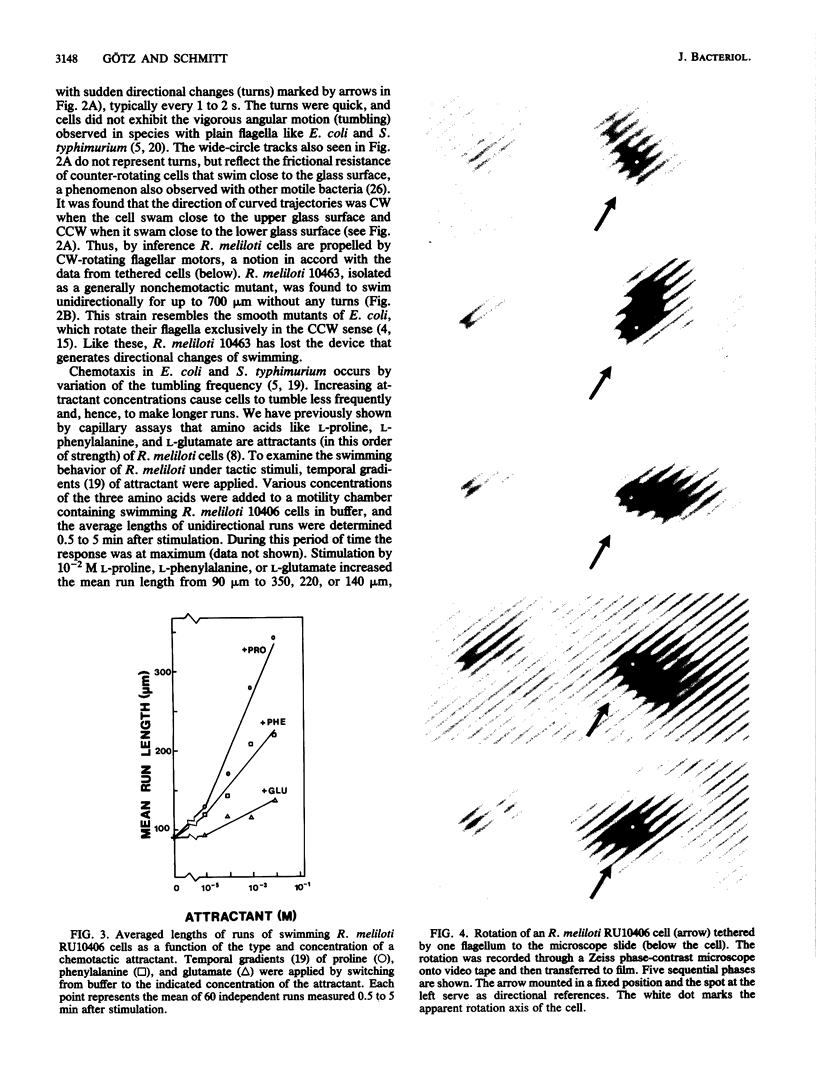
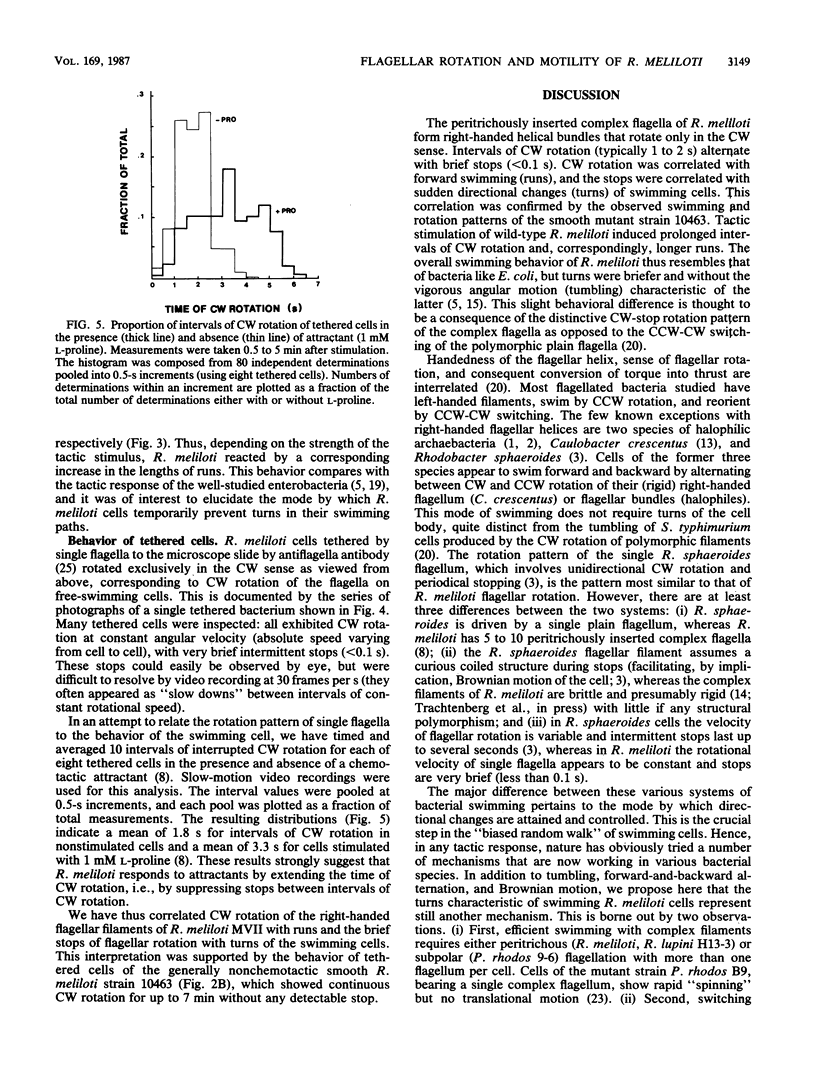
The 5 to 10 peritrichously inserted complex flagella of Rhizobium meliloti MVII-1 were found to form right-handed flagellar bundles. Bacteria swam at speeds up to 60 microns/s, their random three-dimensional walk consisting of straight runs and quick directional changes (turns) without the vigorous angular motion (tumbling) seen in swimming Escherichia coli cells. Observations of R. meliloti cells tethered by a single flagellar filament revealed that flagellar rotation was exclusively clockwise, interrupted by very brief stops (shorter than 0.1 s), typically every 1 to 2 s. Swimming bacteria responded to chemotactic stimuli by extending their runs, and tethered bacteria responded by prolonged intervals of clockwise rotation. Moreover, the motility tracks of a generally nonchemotactic ("smooth") mutant consisted of long runs without sharp turns, and tethered mutant cells showed continuous clockwise rotation without detectable stops. These observations suggested that the runs of swimming cells correspond to clockwise flagellar rotation, and the turns correspond to the brief rotation stops. We propose that single rotating flagella (depending on their insertion point on the rod-shaped bacterial surface) can reorient a swimming cell whenever the majority of flagellar motors stop.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam M., Claviez M., Oesterhelt D., Kessel M. Flagella and motility behaviour of square bacteria. EMBO J. 1984 Dec 1;3(12):2899–2903. doi: 10.1002/j.1460-2075.1984.tb02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Oesterhelt D. Morphology, function and isolation of halobacterial flagella. J Mol Biol. 1984 Jul 15;176(4):459–475. doi: 10.1016/0022-2836(84)90172-4. [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Macnab R. M. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J Bacteriol. 1987 Feb;169(2):514–518. doi: 10.1128/jb.169.2.514-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Tedesco P. M. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Simon M. Bacterial chemotaxis. Annu Rev Physiol. 1982;44:501–517. doi: 10.1146/annurev.ph.44.030182.002441. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Hotani H. Micro-video study of moving bacterial flagellar filaments. III. Cyclic transformation induced by mechanical force. J Mol Biol. 1982 Apr 25;156(4):791–806. doi: 10.1016/0022-2836(82)90142-5. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Segall J. E., Block S. M., Berg H. C. Coordination of flagella on filamentous cells of Escherichia coli. J Bacteriol. 1983 Jul;155(1):228–237. doi: 10.1128/jb.155.1.228-237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., Shirakihara Y. Caulobacter crescentus flagellar filament has a right-handed helical form. J Mol Biol. 1984 Feb 15;173(1):125–130. doi: 10.1016/0022-2836(84)90407-8. [DOI] [PubMed] [Google Scholar]
- Krupski G., Götz R., Ober K., Pleier E., Schmitt R. Structure of complex flagellar filaments in Rhizobium meliloti. J Bacteriol. 1985 Apr;162(1):361–366. doi: 10.1128/jb.162.1.361-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Aizawa S. Bacterial motility and the bacterial flagellar motor. Annu Rev Biophys Bioeng. 1984;13:51–83. doi: 10.1146/annurev.bb.13.060184.000411. [DOI] [PubMed] [Google Scholar]
- Macnab R. M. Examination of bacterial flagellation by dark-field microscopy. J Clin Microbiol. 1976 Sep;4(3):258–265. doi: 10.1128/jcm.4.3.258-265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Han D. P. Asynchronous switching of flagellar motors on a single bacterial cell. Cell. 1983 Jan;32(1):109–117. doi: 10.1016/0092-8674(83)90501-9. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Lodderstaedt G., Schmitt R. Purification and biochemical properties of complex flagella isolated from Rhizobium lupini H13-3. Biochim Biophys Acta. 1978 Jul 21;535(1):110–124. doi: 10.1016/0005-2795(78)90038-7. [DOI] [PubMed] [Google Scholar]
- Schmitt R., Raska I., Mayer F. Plain and complex flagella of Pseudomonas rhodos: analysis of fine structure and composition. J Bacteriol. 1974 Feb;117(2):844–857. doi: 10.1128/jb.117.2.844-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Kamiya R., Asakura S. Left-handed to right-handed helix conversion in Salmonella flagella. Nature. 1975 Mar 27;254(5498):332–334. doi: 10.1038/254332a0. [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Koshland D. E., Jr Reversal of flagellar rotation in monotrichous and peritrichous bacteria: generation of changes in direction. J Bacteriol. 1974 Aug;119(2):640–642. doi: 10.1128/jb.119.2.640-642.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J., Aizawa S., Macnab R. M. Pairwise perturbation of flagellin subunits. The structural basis for the differences between plain and complex bacterial flagellar filaments. J Mol Biol. 1986 Aug 20;190(4):569–576. doi: 10.1016/0022-2836(86)90242-1. [DOI] [PubMed] [Google Scholar]