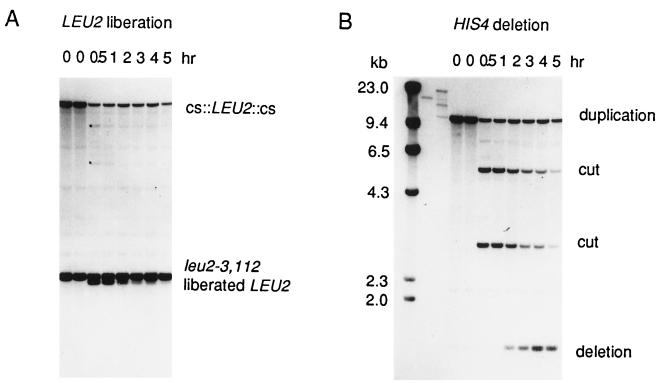
Figure 2.
Physical monitoring of the liberation of a LEU2 fragment and the repair of the broken chromosome by single-strand annealing. Strain G304, carrying the his4′–URA3–cs::LEU2::cs–′his4 insertion illustrated in Fig. 1 and the TRP1, GAL1::HO centromere plasmid pFH800 was grown in YEP-lactate to which 2% galactose (final concentration) was added at t = 0 hr. DNA was extracted at intervals, purified, and cleaved with restriction enzymes for Southern blot analysis. (A) The liberation of the LEU2 fragment is seen on a HpaI–SnaBI digest of DNA, probed with a LEU2-specific probe. Virtually all of the DNA that is cleaved by HO endonuclease is cut at both flanking cut sites to produce a 2.1-kb fragment. Two very faint bands of 7.4 kb and 5.0 kb, marked by dots in the 0.5-hr lane, are the restriction fragments expected for cleavage at only one site. The liberated LEU2 fragment is visible for more than 2 hr before it is apparently degraded. (B) The HO endonuclease-cleaved chromosomal DNA efficiently recombines to form a His+ recombinant. HpaI–SnaBI-digested DNA, probed with a HIS4-specific probe, initially reveals a 10.2-kb restriction fragment containing the entire his4′–URA3–cs::LEU2::cs–′his4 region. HO endonuclease cleavage produces two fragments of 5.3 and 2.9 kb, respectively. After approximately 1 hr, the final His+ recombinant band of 1.4 kb is visible.