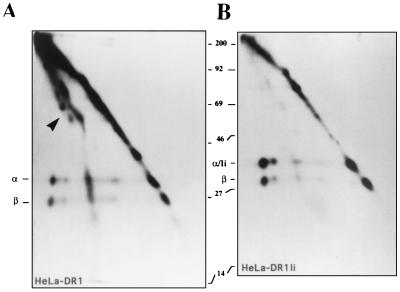
Figure 5.
Comparison of the polypeptides bound to DR molecules immunoprecipitated from Ii+ or Ii- HeLa DR1 cells. DR1 molecules from HeLa DR1 (A) or HeLa DR1/Ii (B) cells were immunoprecipitated using the anti-class II antibody ISCR3 (which binds specifically to intact αβ heterodimers) and run on a two-dimensional SDS/PAGE without boiling in the first dimension (left to right), and boiled in the second dimension (top to bottom). The α and β chain of the DR dimer are easily separated in the second dimension in boiled/reducing conditions. The arrow in A points to a heterogeneous array of polypeptides, which form 1:1 complexes with DR1 dimers in the absence of Ii. The polypeptide/DR1 complexes are shifted to the left of the diagonal because of their SDS stability (A). Ii comigrates with the DR α chain in the second dimension in B. Cells were labeled and immunoprecipitated as described in ref. 21.