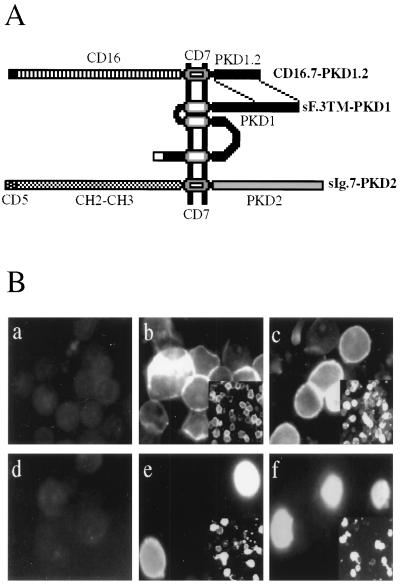
Figure 2.
Construction (A) and surface expression (B) of membrane-bound PKD1 and PKD2 fusion proteins. (A) The C-terminal 113 amino acids of PKD1 (PKD1.2) and the extracellular domain of CD16 were fused to the transmembrane region of CD7 to yield the chimeric integral membrane protein CD16.7–PKD1.2. The construct sF.3TM–PKD1, containing the last three putative transmembrane domains plus the cytoplasmic tail of PKD1, is tagged at its N terminus with the leader sequence of preprotrypsin followed by a FLAG epitope. The membrane-bound fusion of PKD2, sIg.7–PKD2, was generated by fusing the C-terminal 289 amino acids of PKD2 to a cell surface expressed immunoglobulin consisting of the leader sequence of CD5, the CH2 and CH3 domain of human IgG1, and the transmembrane region of CD7. (B) Surface expression of CD16.7–PKD1.2 and sIg.7–PKD2 fusion proteins. 293T cells were transfected with vector (a), CD16.7 (b), CD16.7–PKD1.2 (c), vector (d), sIg.7 (e), sIg.7–PKD2 (f), and labeled with anti-CD16-fluorescein isothiocyanate (a–c) or anti-human IgG-phycoerythrin (d–f). Photographs were taken at ×400 magnification under fluorescence microscopy 24 hr after transfection. (Insets) Lower magnification (×100) are located in the right lower of b, c, e, and f. Expression of CD16.7–PKD1.2 (c) and sIg.7–PKD2 (f) was clearly detected on the surface of unfixed cells.