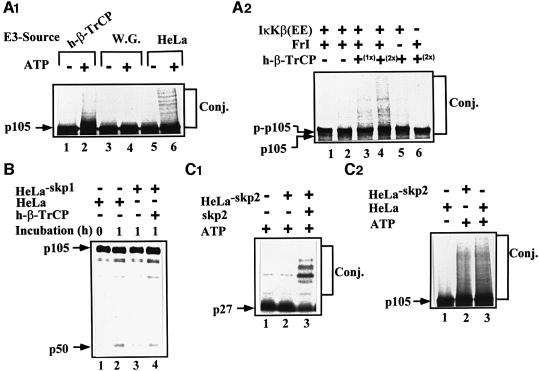
Fig. 8. β-TrCP, but not Skp2, is the ubiquitin ligase involved in conjugation and processing of phosphorylated p105 in vitro. (A1) h-β-TrCP reconstitutes p105 conjugation. 35S-labeled p105 translated in wheat germ extract was subjected to in vitro kinase assay in the presence of IκKβ, and conjugation was carried out as described in Materials and methods. Recombinant and purified SCFh-β-TrCP (lanes 1 and 2), wheat germ extract (75 µg of protein; lanes 3 and 4) or crude HeLa extract (75 µg of protein; lanes 5 and 6) were used as the source of E3. (A2) IκKβ-dependent phosphorylation of p105 is required for its β-TrCP-mediated conjugation. 35S-labeled p105 (as in A1) was subjected to in vitro kinase assay in the presence (lanes 1–5) or absence (lane 6) of IκKβ as described in Materials and methods, and conjugation was monitored as described. Purified recombinant tetrameric SCFβ-TrCP was used at 0.75 (1×; lane 3) and 1.5 (2×; lanes 4 and 6) µg. (B) SCFβ-TrCP reconstitutes processing of p105. 35S-labeled p105 (as in A1) was subjected to in vitro kinase assay as described in Materials and methods. Processing was carried out in a complete (lanes 1 and 2) or Skp1-depleted (lanes 3 and 4) HeLa cell extract in the absence (lane 3) or presence (lane 4) of purified SCFβ-TrCP as described in Materials and methods. (C) Skp2 is not required for conjugation of phosphorylated p105. (C1) Conjugation of p27Kip1 requires Skp2. In vitro trans lated 35S-labeled p27Kip1 was incubated in the presence of Skp2-immunodepleted synchronized HeLa extract as described in Materials and methods. Recombinant and purified Skp2 (0.5 µg) were added when indicated. (C2) Conjugation of p105 is Skp2 independent. 35S-labeled p105 (as in A1) was subjected to in vitro kinase assay in the presence of IκKβ as described in Materials and methods. Conjugation was monitored in the presence of complete or Skp2-immunodepleted HeLa cell extract as indicated and described in Materials and methods. All notes are as described in the legend to Figure 1.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.