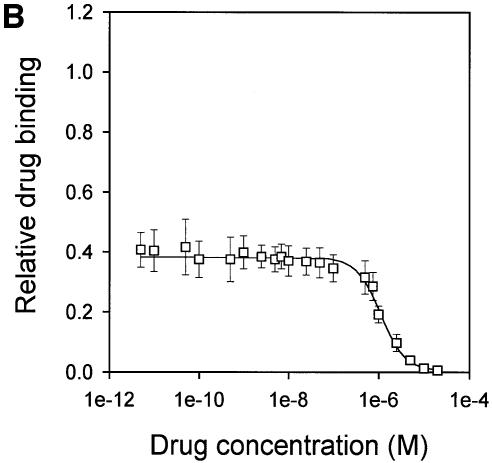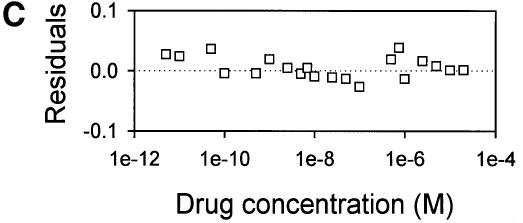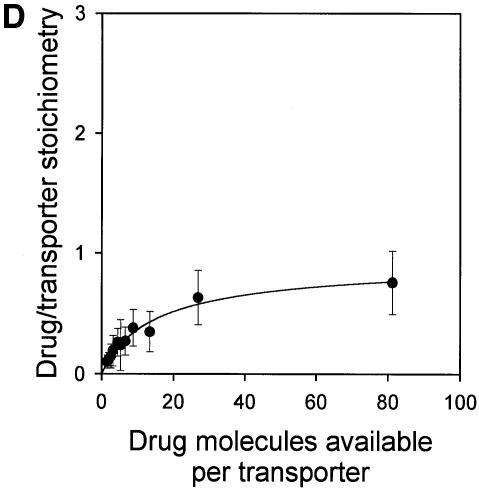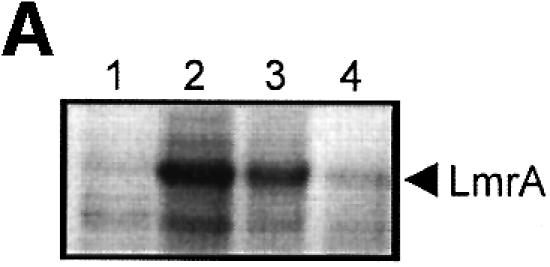
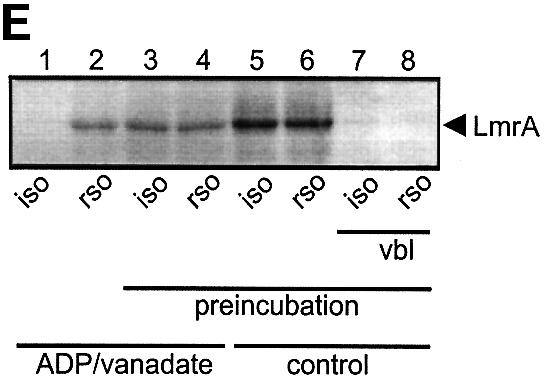
Fig. 6. Vanadate-trapped LmrA contains a single, low-affinity drug-binding site, which is exposed on the outside surface of the membrane. (A) Vanadate-trapped LmrA exhibits reduced affinity for [3H]APDA in photoaffinity labeling experiments. Inside-out membrane vesicles lacking LmrA (lane 1) or containing LmrA (lane 2), vanadate-trapped LmrA (lane 3), or LmrA in buffer supplemented with 3 µM vinblastine (lane 4), were incubated for 2 h at room temperature with 130 nM [3H]APDA. Subsequently, the four samples were UV irradiated, and analyzed by SDS–PAGE and autoradiography. Each lane contained 25 µg of protein. The arrow indicates photolabeled LmrA. (B) Heterologous displacement of [3H]vinblastine by CP100-356 from vanadate-trapped LmrA. Vinblastine displacement was performed as described in the legend to Figure 4C, in the presence of 2 mM Mg-ADP and 2 mM o-vanadate. A relative binding value of 1 represents 1.42 nmol vinblastine/mg of membrane protein. The data suggest the presence of a single vinblastine-binding site in vanadate-trapped LmrA with binding characteristics similar to those of the low-affinity site in the non-trapped protein. (C) Residual variance between the experimental data and the best fitting curve from (B). (D) Direct determination of the vinblastine/LmrA transporter stoichiometry suggests the presence of a single vinblastine-binding site in vanadate-trapped LmrA. Experimental details are similar to those described in the legend to Figure 5, with 2 mM Mg-ADP plus 2 mM o-vanadate included in the vinblastine binding assays. (E) Orientation of the low-affinity drug-binding site in vanadate-trapped LmrA. The accessibility of the low-affinity drug-binding site in vanadate-trapped LmrA from the inside or outside surface of the membrane was determined by photoaffinity labeling with [3H]APDA. LmrA-containing inside-out (ISO) membrane vesicles (lanes 1 and 3) in buffer supplemented with 2 mM Mg-ADP and 2 mM o-vanadate, and LmrA-containing right-side-out (RSO) membrane vesicles loaded with 2 mM Mg-ADP and 2 mM o-vanadate (lanes 2 and 4), were pre-incubated for 2 h at room temperature in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of 130 nM [3H]APDA, and cooled on ice–water. Subsequently, 130 nM [3H]APDA was added to the incubations of lane 1 and 2, and the four reaction mixtures were immediately UV irradiated for 5 min. Non-vanadate-trapped LmrA in inside-out and right-side-out membrane vesicles in the absence (lane 5 and 6, respectively) or presence (lane 7 and 8, respectively) of 5 µM vinblastine were pre-incubated with APDA for 2 h at room temperature prior to UV irradiation. Samples containing 15 µg of membrane protein were analyzed by SDS–PAGE and autoradiography. The arrow indicates photolabeled LmrA.