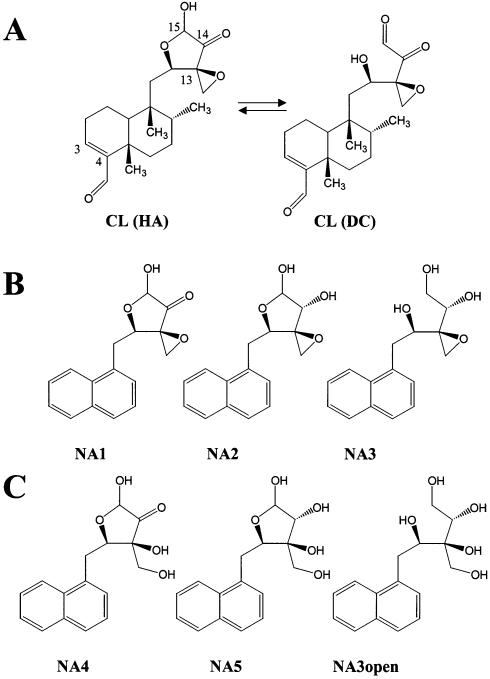
Figure 1.
Structures of CL and NA derivatives. (A) The dicarbonylic form of the drug (DC) is shown in equilibrium with the closed hemi-acetal species (HA). The numbered atoms outline electrophilic carbons possibly involved in reactions with nucleophilic moieties in the DNA. (B) Intact epoxide naphthalene derivatives. (C) Open epoxide naphthalene derivatives. The carbonyl functions in NA1 and NA4 are unmodified, in NA2 and NA5 they are partially reduced, and in NA3 and NA3open they are fully reduced.