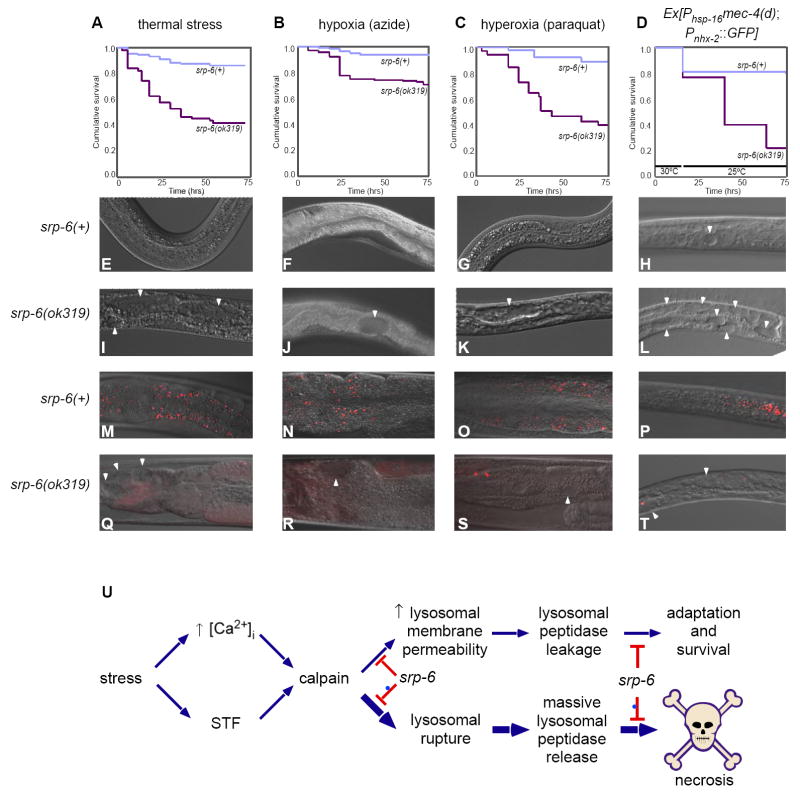
Figure 7. SRP-6 Protected Animals from Different Stressors.
(A-D) Kaplan-Meier curves comparing the survival of srp-6(+) and srp-6(ok319) animals after exposure to (A) thermal stress, (B) hypoxia, (C) hyperoxia or (D) a Phsp-16mec-4(d) transgene. (n ≈ 75 animals/group; P < 0.001, log-rank test).
(E-T) DIC (E-L) and fluorescent (M-T) images of intestinal cell vacuolization (arrowheads) and AO+ intestinal cell lysosomes (arrowheads), respectively, of srp-6(+) (E-H and M-P) and srp-6(ok319) animals (I-L and Q-T) exposed the stressor indicated above the survival curve.
(E) Hypothetical core stress response pathway regulated by SRP-6. Different stressors converge on a core stress response pathway that triggers an increase in [Ca2+]i and modulation of at least one other stress-transducing factor (STF). Cytosolic Ca2+ and STF activate calpains, which associate with lysosomal membranes and enhance the lysosomal response to stress by facilitating, for example, autophagy. Calpains also increase lysosomal membrane permeability allowing for the small leakage of cysteine peptidases into the surrounding cytosol. Cytosolic cysteine peptidases also could provide an adaptive function by enhancing, for example, cytoskeletal rearrangements. However, in the absence of SRP-6, excessive calpain activity leads to massive lysosomal rupture, overwhelming release of unregulated lysosomal cysteine peptidases and necrotic cell death.